METHOD OF PREPARATION
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Mix the thiabendazole with the glycerin until smooth and uniform.
4. Geometrically incorporate the hydrophilic ointment and mix until uniform.
5. Package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed.
STABILITY
A beyond-use date of 30 days can be used for this preparation.1
USE
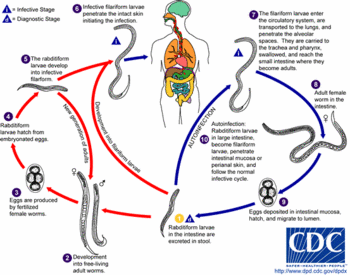
Thiabendazole tropical cream has been used in the treatment of cutaneous larva migrans.
QUALITY CONTROL
Quality-control assessment can include theoretical weight compared to actual weight, specific gravity, active-drug assay, color, texture-surface, texture-spatula spread, appearance, feel, rheological properties and physical observations.2
DISCUSSION
Thiabendazole (C^sub 10^H^sub 7^N^sub 3^S, MW 201.25) occurs as a white to practically white, odorless or practically odorless powder. It is practically insoluble in water, slightly soluble in acetone and alcohol and very slightly soluble in ether. It melts between 296° and 303°C. It is official as an oral suspension and as oral tablets. Thiabendazole is an anthelmintic used in the treatment of strongyloidiasis, cutaneous larva migrans, visceral larva migrans, dracunculiasis, trichinosis and mixed helminthic infections. An unlabeled use is in the treatment of cutaneous larva migrans.1,3
Glycerin (C^sub 3^H^sub 8^O^sub 3^, MW 92.1, glycerol, 1,2,3-propane triol) occurs as a clear, colorless, odorless, viscous, hygroscopic liquid with a sweet taste about two thirds as sweet as that of sucrose. It is used as an antimicrobial preservative (>20% concentration), as an emollient and humectant (up to 30% concentration), in ophthalmic formulations (0.5% to 3% concentration), as a plasticizer in film coating for tablets, as a parenteral solvent (up to 50% concentration) and as a sweetening agent in alcoholic elixirs (up to 20% concentration). It has a specific gravity of about 1.25 and a melting point of 17.8°C; if cooled to crystallization, it will need to be heated to about 20°C to melt. It is miscible with water, methanol and 95% ethanol, practically insoluble in oils and chloroform and slightly soluble in acetone. It is hygroscopic and should be stored in airtight containers in a cool place. It is not prone to oxidation but will decompose on heating. When mixed with water, ethanol and propylene glycol, the mixtures are chemically stable.4
Hydrophilic ointment is a water-washable oil-in-water emulsion base containing methylparaben, propylparaben, sodium lauryl sulfate, propylene glycol, stearyl alcohol, white petrolatum and purified water. It is miscible with water and aqueous solutions and some amount of oil solutions can be incorporated into the inner phase of the emulsion.5,6
Dermabase is an unscented, washable, oil-in-water emulsion cream base. It contains purified water (about 45%), mineral oil, petrolatum, cetostearyl alcohol, propylene glycol, sodium lauryl sulfate, isopropyl palmitate, imidazolidinyl urea, methylparaben and propylparaben. It is a smooth, white, water-washable cream with a slight, pleasant odor. It is preserved and is compatible with a wide variety of agents.7
Vanicream is an oil-in-water emulsion base containing white petrolatum, cetearyl alcohol, ceteareth-20, sorbitol, propylene glycol, simethicone, glyceryl monostearate, polyethylene glycol monostearate and sorbic acid. It is free of dyes, perfume, lanolin, parabens and formaldehyde and is a stable and widely compatible cream.8
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary 22. Rockville, MD: US Pharmacopeial Convention, Inc.; 2004: 2345-2349, 2785.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC1998; 2(4): 308-309.
3. Lacy CF, Armstrong LL, Goldman MP et al. Lexi-Comp's Drug Information Handbook. 12th ed. Hudson, OH: Lexi-Comp; 2004:1403-1404.
4. Price JC. Glycerin. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 257-259.
5. Reilly WJ Jr. Pharmaceutical necessities. In: Gennaro AR, ed. Remington: The Science and Practice of Pharmacy. 19th ed. Easton, PA: Mack Publishing Company; 1995:1402.
6. Rlock LH. Medicated applications. In: Gennaro AR, ed. Remington: The Science and Practice of Pharmacy. 19th ed. Easton, PA: Mack Publishing Company; 1995:1586.
7. Dermabase [product information]. Minneapolis, MN: Paddock Laboratories, Inc.
8. Vanicream [product information]. Rochester, MN: Pharmaceutical Specialties, Inc.
Copyright International Journal of Pharmaceutical Compounding Mar/Apr 2005
Provided by ProQuest Information and Learning Company. All rights Reserved