Wolff-Parkinson-White syndrome is the most common of the preexcitation syndromes and is due to an accessory pathway connecting the atria and ventricles. Asymptomatic patients do not require empirical therapy. Ongoing drug therapy has traditionally been the choice for older symptomatic patients and patients with infrequent symptoms. However, more definitive therapy, ie, surgical or nonsurgical ablation, may be preferable for younger patients, patients with medically refractory arrhythmias, and patients at high risk for sudden death. Ablation by radiofrequency current is now being routinely performed because it appears to be cost-effective and has been found to be safe and efficient if performed by skilled operators. Clinical experience of this procedure is limited, however, and long-term effects still need to be established.
Key words. Wolf-Parkinson-White syndrome; catheter ablation. (J Fam Pract 1995; 41:497-500)
Many recent advances have explained the mechanism of preexcitation syndromes and the treatment of associated rhythms. Wolf-Parkinson-White (WPW) syndrome, the most common of the preexcitation syndromes, is characterized by the presence of an accessory conduction pathway between the atrium and the common ventricular tissue.[1] Although many treatment options are available, transcatheter radiofrequency ablation has increasingly become the therapy of choice for safe and definitive management of patients with WPW syndrome.
Case Study
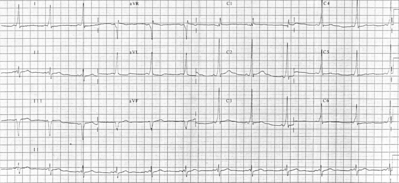
A 20-year-old man was admitted with complaints of palpitations beginning a week before admission. Initially, palpitations were daily, increasing in frequency to three times a day, lasting approximately 5 minutes per episode. One episode was eventful and included 15 to 20 minutes of lightheadedness and nausea. The patient denied any other symptoms, such as dyspnea, diaphoresis, or syncope. His past medical history was unremarkable, and he denied taking any medications or recreational drugs. Following admission, he developed a spontaneous arrhythmia of a narrow QRS tachycardia (Figure 1). Valsalva and carotid massage techniques were ineffective. He was then given a loading dose followed by intravenous procainamide hydrochloride at 2 mg per minute, which restored his sinus rhythm. Following restoration of sinus rhythm, 12-lead electrocardiogram (ECG) readings revealed a decreased PR interval (0.10 seconds), a widened QRS complex (0.12 seconds), and a slurring of the initial upstroke of the QRS consistent with a delta wave (Figure 2). Physical examination findings were unremarkable and results of serum electrolytes measurement, complete blood count, thyroid function tests, and urinalysis were normal. A two-dimensional echocardiogram showed no structural abnormalities. The clinical presentation and ECG findings were consistent with the diagnosis of WPW syndrome.
The patient was informed of treatment options for WPW syndrome, including ongoing pharmacological therapy, surgical ablation, and radiofrequency catheter ablation. He preferred a diagnostic electrophysiologic study with radiofrequency ablation. In the electrophysiologic laboratory, four multiple electrode catheters were positioned in the appropriate cardiac chambers under fluoroscopic guidance. Programmed stimulation of the atria and ventricles was performed to identify the location of the bypass tract. The test revealed a single left lateral accessory pathway. The pathway was ablated using a large-tipped catheter connected to a radiofrequency lesion generator system, which delivered a radiofrequency at the earliest ventricular activation site during sinus rhythm. Twenty to 50 watts of power was applied seven times for 10 to 20 seconds to successfully ablate the bypass tract. Subsequent ECG revealed normalization of the PR and QRS intervals and the absence of delta waves (Figure 3). The patient suffered no complications from the procedure and was discharged later the same evening. At follow-up 4 months later, he had had no recurrence of symptoms, and repeat ECG revealed no evidence of preexcitation.
Discussion
Wolf-Parkinson-White syndrome is a congenital abnormality with a prevalence of 0.15% to 0.3%.[1,2] The presence of an accessory pathway (AP) between the atrial and ventricular tissue allows atrial impulses to bypass the normal conduction pathway of the heart and depolarize the ventricles early, which produces a circuit capable of sustaining tachyarrhythmias. The characteristic ECG findings include a short Printerval, a wide QRS complex, and the presence of a delta wave. The PR interval is short 0.12 second) owing to the electrical impulse bypassing the normal delay occurring at the atrioventricular (AV) node and rapidly stimulating the ventricles. This premature ventricular depolarization causes the formation of a delta wave, which is a slurring of the initial QRS upstroke. The QRS complex is wide (>0.10 second) because of the preexcitation of one ventricle and the asynchronous depolarization of both ventricles. Symptoms develop in approximately 25% to 50% of all patients with WPW syndrome and may include palpitations, diaphoresis, light-headedness, dizziness, nausea, vomiting, and syncope.[3] Wolf-Parkinson-White syndrome is also associated with congenital heart diseases such as Ebstein's anomaly, tricuspid atresia, septal defects, and transposition of the great vessels.
The most common form of tachyarrhythmias associated with WPW syndrome is AV reciprocating tachycardia, of which there are two types: orthodromic and antidromic.[3] In orthodromic tachycardia, the atrial impulse conducts in an antegrade direction from the atria through the AV node and bundle of His to the ventricles, producing a narrow QRS complex. The impulse then travels retrograde through the AP to the atria, and the cycle is repeated. Antidromic tachycardia is conducted antegrade through the AP to the ventricles, and then retrograde back to the atria via the AV node. A wide QRS and prominent delta wave are characteristic of antidromic tachycardia. Approximately 84% of patients with symptomatic WPW have orthodromic tachycardia.
Atrial fibrillation is the second most common arrhythmia in patients with symptomatic WPW, with an incidence of 11.5%.[4] This may degenerate in to ventricular fibrillation owing to rapid antegrade conduction through the AP if the refractory period is short. Persons with a short RR interval (<0.25 second) during induced atrial fibrillation or with a short antegrade effective refractory period of the AP (<0.27 second) appear to be at increased risk for sudden death.[5]
The hemodynamic status of the patient and the mechanism of the arrhythmia determine the therapeutic approach to tachyarrhythmic episodes.[3] Vagal maneuvers (carotid sinus massage, Valsalva maneuver) should be the first therapeutic intervention if the patient has a narrow complex tachycardia and is stable. If unsuccessful, intravenous adenosine or verapamil hydrochloride may be given to slow AV conduction. If digoxin or verapamil is used in wide complex tachycardias, it should be used with caution because both slow conduction through the AV node but may increase conduction through the AP, causing enhancement of conduction to the ventricles. Procainamide infusion is the drug of choice in this case because it is able to reduce conduction through the AP. However, in an unstable or medically refractory patient, cardioversion is indicated.
Long-term treatment options include no therapy, pharmacologic prophylaxis, and surgical or catheter ablation. No therapy is the appropriate approach only for asymptomatic patients or patients with mild symptoms that do not interfere with activities of daily living. Antiarrhythmic prophylaxis is usually lifelong and should be considered, especially in older symptomatic patients. Drugs most often used are those in classes IA, IC, III, and beta blockers, and include procainamide, propafanone, flccainide, amiodarone hydrochloride, nadolol, and sotalol.
More definitive ablative therapy is recommended for younger patients who have proarrhythmic potential and for whom drug therapy would be lifelong; patients with arrhythmias refractory to pharmacologic therapy; and patients at high risk for sudden death.6 Surgical ablative therapy, which involves either endocardial dissection of the AP or epicardial dissection and cryoablation of the AP in the AV groove, is one alternative. Ablation of bypass tracts in two reported studies was successful in 81% and 99% of patients, with higher failure rates in children.[7,8] Overall mortality ranged from 4.1% to 5%, but the majority of these patients had preexisting cardiac abnormalities. Complications were minimal, but included postpericardiotomy syndrome, temporary right ventricular dysfunction, temporary low output failure, postoperative atrial fibrillation, complete heart block, postoperative hemorrhage, tricuspid insufficiency, and constrictive pericarditis.
Although the surgical approach to WPW syndrome is associated with high efficacy, it requires an open thoracotomy and is also associated with relatively high mortality. Nonsurgical direct current (DC) catheter ablation of the AV junction in patients with supraventricular tachycardia was documented in 1982.[9,10] High-energy electrical shocks are delivered to the tissue by a percutancous catheter and produce barotrauma and necrosis. Successful DC catheter ablation has been reported in 75% to 90% of cases.[11,12] This technique is associated with infrequent but serious complications, including cardiac perforation, coronary artery spasm, cardiogenic shock, AV block, and delayed ventricular fibrillation.
In 1987, the first clinical use of radiofrequency (RF) energy for catheter ablation of an AP was reported.[13] In this process, unmodulated PF current is delivered through a catheter to the tissue, causing the acceleration of cellular ions and resistive heating. This results in coagulation necrosis producing a small, accurate lesion with discrete borders. Radiofrequency ablation is superior to DC ablation, which produces a larger and less precise lesion; RF ablation also is associated with fewer cardiac arrhythmias and fewer complications, does not require general anesthesia, and requires less hospitalization time.[14,15] Because of its high frequency, the RF current does not stimulate neuromuscular fibers. Up to 40% of patients, however, have been reported to experience mild chest pain during the administration of the R-F current. In four major studies,[14,16-18] successful ablation rates have ranged from 53% to 99%, depending on the location of the AP. There was a total of 527 patients in all four studies, with an average success rate of 90.1% and no reported mortalities. A total of 17 complications was reported, with an average complication rate of 3.2%. Complications include AV block, pericarditis, cardiac tamponade, acute myocardial infarction, cerebral embolism, air embolism, wall perforation, arteriovenous fistula at groin puncture site, and arterial thrombosis.
As clinical experience with RF ablation has increased, many cardiologists now believe it is the treatment of choice for all symptomatic WPW patients. However, with increased acceptance, less experienced teams may perform the procedure, which may increase the complication rate. Additionally, no long-term follow-up results are yet available. The recurrence rate of tachycardia after several years remains to be seen. Although the risk of acute occlusion of coronary arteries is very small, long-term follow-up is needed to determine whether RF lesions promote the development of coronary atherosclerosis.[15] Surgical ablation is still indicated for patients who have failed catheter ablation, have required emergency operation during attempted catheter ablation, or have associated structural heart disease requiring concomitant surgical correction. Attempted catheter ablation does not appear to adversely affect the safety and efficacy of operative therapy.[19]
Figures 1 to 3 [ILLUSTRATION OMITTED]
References
[1.] Vidaillet HJ, Pressly JC, Henke E, et al. Familial occurrence of accessory atrioventricular pathways (preexcitation syndrome). N Engl J Med 1987; 317:65-9. [2.] Fisch C. Clinical electrophysiology studies and the Wolff-Parkinson-White pattern. Circulation 1990; 82:1872-3. [3.] Berry VA. Wolff-Parkinson-White syndrome and the use of radio-frequency catheter ablation. Heart Lung 1993; 22(1):15-25. [4.] Wellens HJ, Durrer D. Wolff-Parkinson-White syndrome and atrial fibrillation: relation between refractory period of accessory pathway and ventricular rate during atrial fibrillation. Am J Cardiol 1974; 34:777-82. [5.] Klein GJ, Badshore TM, Sellers TD, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med 1979; 301: 1080-5. [6.] Singer I, Kupersmith J. Nonpharmacological therapy of supraventricular arrhythmias: surgery and catheter ablation techniques, part 2. PACE Pacing Clin Electrophysiol 1990; 13:1173-83. [7.] Gallagher JJ, Sealy WC, Cox JL, et al. Results of surgery, for preexcitation caused by accessory atrioventricular pathways in 267 consecutive cases. In: Josephson ME, Wellens HJJ, eds. Tachycardias: mechanisms, diagnosis, treatment. Philadelphia, Pa: Lea & Febiger, 1984:259. [8.] Cox JL, Gallagher JJ, Cain ME. Experience with 118 consecutive patients undergoing operation for the Wolff-Parkinson-White syndrome. J Thorac Cardiovasc Surg 1985: 90:490-501. [9.] Scheinman MM, Morady F, Hess DS, et al. Catheter-induced ablation of atrioventricular junction to control refractory supraventricular arrhythmias. JAMA 1982; 248:8 5 1. [10.] Gallagher JJ, Svenson RH, Kasell JH, et al. Catheter technique for closed chest ablation of the atrioventricular conduction system: a therapeutic alternative for the treatment of refractory, supraventricular tachycardia. N Engl J Med 1982; 306:194. [11.] Morady P, Scheinman MM, Winston SA, et al. Efficacy and safety of transcatheter ablation of posteroseptal accessory pathways. Circulation 1985; 72(1):170-7. [12.] Warin JF, Haissaguerre M, Lemetayer P, et al. Catheter ablation of accessory pathways with a direct approach (results in 35 patients). Circulation 1988; 78: 800-15. [13.] Borggrefe M, Hindricks G, Haverkamp W, et al. Catheter ablation using radiofrequency energy. Clin Cardiol 1990; 13:127-31. [14.] Jackman WM, Wang X, Friday KJ, et al. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med 199 1; 324:1605-11. [15.] Barrington WW, Greenfield RA, Bacon ME, ct al. Radiofrequency ablation of SVT. Am J Med 1992; 93:549-57. [16.] Calkins H, Sousa J, El-Atassi R, et al. Diagnosis and cure of the Wolff-Parkinson-White syndrome or paroxysmal supraventricular tachycardias during a single electrophysiologic test. N Engl J Med 1991; 324:1612-8. [17.] Thakur RK, Klein GJ, Yee R. Radiofrequency catheter ablation in patients with Wolff-Parkinson-White syndrome. Can Med Assoc J 1994; 151:771-6. [18.] Kuck K, Shluter M, Geiger M, et al. Radiofrequency current catheter ablation of accessory atrioventricular pathways. Lancet 1991; 337:1557-61. [19.] Guiraudon GM, Guiraudon CM, Klien GJ, et al. Operation for the Wolff-Parkinson-White syndrome in the catehter ablation era. Ann Thorac Surg 1994; 57:1084-8.
Submitted, revised, August 2, 1995.
From the University of Michigan Medical Center, Ann Arbor, Michigan. Requests for reprints should be addressed to Melvyn Rubenfire, MD, University of Michigan Medical Center, 9D University Hospital/9800, 1500 East Medical Center Dr, Ann Arbor, MI 48109-0119.
COPYRIGHT 1995 Dowden Health Media, Inc.
COPYRIGHT 2004 Gale Group