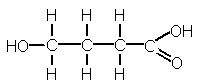
Brussels, Belgium - December 8, 2005: UCB announced today that
Xyrem® (sodium oxybate) oral solution is now available in Germany for
the treatment of cataplexy in adult patients with narcolepsy. Launch
in this first major European market follows the recent European
Commission (EC) marketing approval of Xyrem® in this orphan
indication, with pan-European commercialisation expected over 2006.
"Xyrem® provides physicians and patients in Europe with the first and
only EMEA approved medication for the treatment of cataplexy in
patients with narcolepsy. We are delighted to serve European
physicians and sleep centres with a product that can help fulfil the
unmet medical need in patients with this serious sleep disorder."
said Emmanuel Caeymaex, Vice-President, Marketing CNS, UCB.
Narcolepsy is a debilitating, life-long neurological disorder that is
characterised by excessive daytime sleepiness and sleep attacks[1].
Cataplexy is a typical symptom of narcolepsy and is present in 65-70%
of patients with narcolepsy. It involves a sudden reversible loss of
muscle tone, usually triggered by emotional stimuli such as laughter,
excitement, surprise and anger, with contributing factors including
physical fatigue, stress or sleepiness[2].
Commenting on the marketing approval Adrian Williams MD of Sleep
Centre, St. Thomas Hospital, London, UK said "Patients and physicians
in the EU should be encouraged by the body of clinical research
supporting Xyrem®. In Europe, the approval of Xyrem® for the
treatment of cataplexy in patients with narcolepsy provides a welcome
addition to the pharmacological armamentarium."
Notes to Editor
1. UCB acquired the licence to distribute Xyrem® in Europe from
Orphan Medical recently acquired by Jazz Pharmaceuticals). Xyrem® is
marketed in the U.S. by Jazz Pharmaceuticals and has been available
in the U.S. since 2002.
2. Orphan medicinal product designation
Xyrem® was designated as an orphan medicinal product on February 3,
2003. Orphan medicinal products are used to diagnose, prevent or
treat life-threatening or very serious conditions that are rare, with
a prevalence of less than five per 10,000 of the EU population.
European orphan drug designation enables recipient sponsors to
receive regulatory guidance in the drug development process and
allows for up to 10 years of European market exclusivity for the
designated indication upon approval of the market application.
3. Clinical trials
The European marketing approval of Xyrem® was mainly based on a
prospective, multicentre, randomized, double-blind,
placebo-controlled trial that examined the safety and efficacy of
Xyrem® (3 g, 6 g and 9 g) for the treatment of narcolepsy symptoms.
Xyrem® was taken in divided nightly doses immediately before
bed-time and repeated 2oe-4 hours later. Results of this four-week
study showed that in comparison to placebo, Xyrem® demonstrated
clinical improvements in the reported number of weekly cataplexy
attacks and daytime sleepiness. Investigators assessed changes in
disease severity using the Clinical Global Impression of change
(CGI-c) measure, and Xyrem® (9 g) was found to significantly decrease
the frequency of cataplexy attacks compared to placebo[1].
A follow-up, 12-month open-label study showed that Xyrem® (3-9 g) was
well-tolerated and produced significant and long-term clinical
improvement in the frequency of cataplexy attacks and diminished
day-time sleepiness[3].
About UCB
UCB (www.ucb-group.com) is a global biopharmaceutical leader with
headquarters in Brussels, Belgium, specialising in the fields of
central nervous system disorders, inflammatory diseases, and
oncology. UCB key products are Keppra® (antiepileptic), Xyzal® and
Zyrtec® (antiallergics), Nootropil® (cerebral function regulator),
Tussionex® (antitussive) and Metadate(TM) / Equasym XL(TM) (attention
deficit/hyperactivity disorder). UCB employs over 8,500 people
operating in over 40 countries. UCB is listed on Euronext Brussels
(UCB / UCBBt.BR / UCB BB).
[1] The U.S. Xyrem® Multicenter Study Group A randomized,
double-blind, placebo controlled multi-centre trial comparing the
effects of three doses of orally administered sodium oxybate with
placebo for the treatment of narcolepsy Sleep 2002: 25 (1), 42-49
[2]
http://www.sleepfoundation.org/sleeptionary/index.php?id=12&subsection=symptomsaccessed
November
6, 2005
[3] The U.S. Xyrem® Multicenter Study Group A 12-month, Open-Label,
Multicenter Extension Trial of Orally Administered Sodium Oxybate for
the Treatment of Narcolepsy Sleep 2003: 26 (1), 31-35
Copyright © Hugin ASA 2005. All rights reserved.