Xyrem
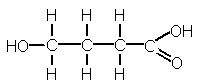
Gamma-hydroxybutyrate (4-hydroxybutanoic acid, C4H8O3) is both a drug and a naturally occurring compound found in the central nervous system as well as in other organs as liver, kidneys, heart and bones. GHB is structurally similar to the ketone body beta-hydroxybutyrate. As a drug it is used most commonly in the form of a chemical salt (Na-GHB or K-GHB). The sodium salt is commercially known as sodium oxybate. more...
Uses
Endogenous
The precise function of GHB in body is not clear. It is an immediate precursor to GABA, a neurotransmitter which regulates awakeness, physical activity and sleep. As GABA cannot cross blood-brain barrier, GHB obtained from food may be used for converting to GABA. GHB prevents cells from oxygen starvation, which might explain presence of the compound in vital organs. GHB was also found to have neuroprotective capabilities.
Medical
It has been used as a general anesthetic, and a hypnotic in the treatment of insomnia. GHB has also been used to treat clinical depression, and improve athletic performance. In the United States, the Food and Drug Administration permits the use of GHB under the trade name Xyrem to reduce the number of cataplexy attacks in patients with narcolepsy.
Recreational
GHB is an intoxicant. It may be known as G, Liquid X, Liquid E. It is less commonly known as GBH, Gamma-oh, Georgia Homeboy, Blue Verve, Gamma-G, Qi, scoop, goop, Grievous Bodily Harm, or Get Hospitalised Bitch.
Its potential for use as a date rape drug in the 1990s led to it being placed in the US on Schedule I of the Controlled Substances Act in March, 2000. On March 20, 2001, the Commission on Narcotic Drugs placed GHB in Schedule IV of the 1971 Convention on Psychotropic Substances. In the UK it was made a class C drug in June 2003.
The sodium salt of GHB has a thin, very salty, chemical taste. At low doses, GHB can cause a state of euphoria, increased sociality and intoxication. This kind of use is particularly common at rave parties. At higher doses, GHB may induce nausea, dizziness, drowsiness, visual disturbances, depressed breathing, amnesia and unconsciousness. The effects of GHB can last from 1.5 to 3 hours.
Some chemicals convert to GHB in the stomach and blood. GBL, or gamma-butyrolactone, is one such precursor. It is 1,6 times more potent than GHB, so 1ml of GBL is equivalent to 0,4g of GHB. GBL has also a shorter onset and is longer acting than GHB. GBL has an extremely bad taste and is also known to irritate innards and skin.
Other precursors include 1,4-butanediol. There may be additional toxicity concerns with these precursors.
Mode of action
The action of GHB has yet to be fully elucidated. GHB clearly has at least two sites of action, stimulating the newly characterized and aptly named "GHB receptor" as well as the GABAB. GHB, if it is indeed a neurotransmitter, will only reach concentrations high enough to act at the GHB receptor, as it only has weak affinity fo the GABAB. However, during recreational usage, GHB can reach very high concentrations in the brain, relative to basal levels, and can act at the GABAB receptor . GHBs action at the GABAB is probably responsible for its sedative effects. GHB-mediated GABAB receptor stimulation inhibits dopamine release as well as causes the release of natural sedative neurosteroids (like all other GABAB agonists e.g. Baclofen). In animals GHBs sedative effects can be stopped by GABAB antagonists (blockers).
Read more at Wikipedia.org