Hypertrophic obstructive cardiomyopathy (HOCM) is an obstructive subvariety of hypertrophic cardiomyopathy. (1) In hypertrophic cardiomyopathy, the left ventricular wall of the heart is abnormally thick, and the left ventricular cavity is small. HOCM is characterized by an excessively thick interventricular septum, which obstructs left ventricular outflow during ventricular systole. (1) Pericardial tamponade is a potential complication after insertion of a pacemaker and can severely worsen obstruction of left ventricular outflow in patients with HOCM. In this article, we present a case study illustrating the cascade of complications that can occur after insertion of a pacemaker in a patient with HOCM and review the pathophysiology of this cardiac abnormality.
Case Study
J.D., a 55-year-old man, was admitted to the coronary intensive care unit for management of chest pain that occurred after insertion of a rate-responsive sequential dual-chamber pacemaker. The pacemaker had been inserted because his heart rate could not increase to match his level of exercise. This condition, chronotropic incompetence, was thought to be responsible for the presyncope with exercise that he had experienced for 1 year. He had had hypertrophic cardiomyopathy for 10 years.
Twelve days before admission, he had had a posterior myocardial infarction that required emergent percutaneous coronary angioplasty of the right coronary and left circumflex arteries. Left ventricular end-diastolic pressure at the time of the angioplasty was 12 mm Hg. Findings on an echocardiogram obtained before the myocardial infarction were essentially normal, with evidence of asymmetrical hypertrophy but no diastolic dysfunction. J.D. had a family history of cardiac disease. His father died of sudden cardiac death at age 38, and 3 uncles had a myocardial infarction before the age of 45 years.
In the coronary intensive care unit, J.D. experienced 2 types of chest pain. The first type, nonradiating chest tightness associated with shortness of breath, was relieved with oxygen therapy and intravenous nitroglycerin and morphine. Administration of intravenous heparin was started. Administration of medications that J.D. had been taking before admission, clopidogrel 75 mg, atorvastatin 20 mg, and aspirin 325 mg, was resumed, and the dose of metoprolol was increased from 50 mg 2 times a day to 75 mg 2 times a day. The second type of pain was a sharp, reproducible, right-sided anterior chest pain, which J.D. described as "muscle spasm in the chest affecting my windpipe" and "it feels like I'm being choked." This pain was not relieved by treatment with nitroglycerin or morphine.
In the coronary intensive care unit, J.D.'s vital signs remained stable: blood pressure 90/60 to 100/70 mm Hg, respirations 12/min to 16/min, and heart rate 66/min to 80/min. Pulsus paradoxus was less than 10 mm Hg, and no pulsus alternans was detected. Findings on electrocardiograms indicated sinus rhythm with first-degree heart block and no acute ST-segment or T-wave changes. Auscultation revealed posterior basal chest crackles. Right jugular venous distention was less than 4 cm above the sternal angle. An [S.sub.4] and a systolic murmur were heard with his heart sounds. Assessment of the pacemaker indicated that the device was functioning normally, pacing and sensing appropriately. The low pacemaker rate was set at 60 beats per minute. Findings on an echocardiogram did not differ from those on the echocardiogram obtained before insertion of the pacemaker.
Throughout the first 2 days after the pacemaker surgery, J.D. intermittently had chest discomfort and anxiety. He said he felt very fatigued, and he had no appetite for food. Treatment with nitroglycerin was discontinued, and no increase in his first type of chest pain occurred. On the second day after surgery, J.D. experienced shortness of breath and increased level of fatigue, his heart sounds became progressively more distant, his heart rate was 90/min, and he became hypotensive, with a blood pressure of 80/50 mm Hg and a paradoxical pulse pressure of 16 mm Hg. His urine output was concentrated and less than 30 mL/h. His nail beds were cyanotic, and his skin was diaphoretic and cool. He was given 40 mg of furosemide, and the rate of intravenous administration of dextrose and isotonic sodium chloride solution was increased to 100 mL/h. Administration of clopidogrel and heparin was discontinued, and protamine was given. Hemoglobin level and hematocrit remained normal, but the white blood cell count increased to 23.6 x [10.sup.9]/L (normal, 4 x [10.sup.9]/L to 11 x [10.sup.9]/L), and the level of serum creatinine increased to 288 [micro]mol/L (normal, 45-100 [micro]mol/L). Both the white blood cell count and the serum creatinine level had been normal at the time of admission to the coronary intensive care unit.
An echocardiogram confirmed a pericardial effusion, which was subsequently drained of 220 mL of serosanguineous fluid. A catheter was placed in the pericardium and was attached to a Jackson-Pratt drainage system. J.D.'s hemodynamic status improved and he felt much better after the pericardiocentesis. He was no longer short of breath and his appetite returned. An echocardiogram showed that the tip of the pacemaker catheter extended through the right ventricular apical wall of the epicardium. Surgery was scheduled for repositioning of the pacemaker.
On the third day after the pacemaker surgery, J.D.'s urine output decreased to less than 10 mL/h despite infusion of dextrose and isotonic sodium chloride solution at 100 mL/h. His response to repeated doses of furosemide was poor. Treatment with low-dose dopamine was started for a renal dilatory effect, but urine output did not improve. Auscultation revealed coarse crackles from the base to the middle of the lungs. With administration of 100% oxygen via a nonrebreather mask, oxygen saturation was 88%.
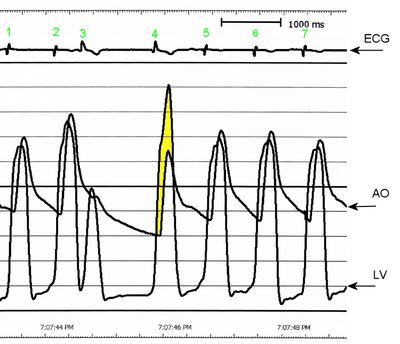
J.D. was taken to the operating room to have his pacemaker catheter repositioned. In the operating room, clotting of the pericardial drain was detected. Patency of the drain was reestablished, and 460 mL of blood was drained. J.D.'s blood pressure increased from 80/50 to 120/50 mm Hg. A transesophageal echocardiogram obtained while he was in the operating room showed obstruction of the left ventricular outflow tract (LVOT), with an outflow gradient of 55 mm Hg. At this time, the diagnosis was expanded to HOCM. After diagnosis of HOCM, J.D. was given metoprolol and verapamil to keep his heart rate less than 60/min and reduce LVOT obstruction. His pacemaker rate was set at 50 beats per minute.
As the third postoperative day after initial insertion of the pacemaker progressed, J.D.'s condition continued to deteriorate. His systolic blood pressure decreased to 60 mm Hg, his respirations were labored at 30/min, and with 100% oxygen delivered via a nonrebreather mask, his oxygen saturation was 85%. His hemoglobin level decreased to 100 g/L (normal, 120-160 g/L). Intravenous infusions of norepinephrine and phenylephrine were started to produce peripheral vasoconstriction and increase blood pressure. An intravenous infusion of esmolol was started, because of the drug's negative inotropic effects, to decrease acceleration of ventricular ejection and thus reduce LVOT obstruction and to keep the heart rate at less than 60/min. J.D. was sedated with midazolam and was intubated for mechanical ventilation. Clotting occurred again in the pericardial catheter, so a pericardial window was created, with good results. Heart sounds remained distant.
An echocardiogram obtained at this time revealed severe systolic anterior motion of the mitral valve leaflet and almost complete LVOT obstruction, resulting in decreased cardiac output and increased mitral regurgitation. The echocardiogram also showed a small right and left ventricular circumferential effusion. A catheter was inserted into the pulmonary artery for hemodynamic monitoring. The hemodynamic readings remained relatively consistent throughout the day: pulmonary artery pressure (PAP) 50/25 mm Hg, with a mean of 30 mm Hg; pulmonary capillary wedge pressure 19 mm Hg; right atrial pressure 20 mm Hg; and cardiac index 1.8 to 2.0 (calculated as cardiac output in liters per minute divided by body surface area in square meters). Blood pressure varied between 90/60 and 120/65 mm Hg throughout the day. Some difficulty occurred in maintaining a consistent systemic vascular resistance index despite the titration of norepinephrine and phenylephrine. The systemic vascular resistance index varied between 1501 and 3204 (calculated as systemic vascular resistance in dynes per second per centimeter to the minus 5 divided by body surface area in square meters), and the pulmonary vascular resistance index was elevated at 625 (calculated as systemic pulmonary resistance in dynes per second per centimeter to the minus 5 divided by body surface area in square meters). Nitric oxide was added to the pressure-support mechanical ventilation circuit to help reduce the pulmonary pressures. Four hours after J.D.'s acute deterioration, spontaneous hemorrhage from the nose developed, possibly because of hepatic ischemia, acute renal failure, or an inflammatory or stress response. The hemoglobin level decreased to 70 g/L. Probable disseminated intravascular coagulopathy was diagnosed. J.D. was given multiple transfusions of fresh-frozen plasma, packed cells, and platelets and injections of vitamin K. His nose was packed. No other sites of bleeding were apparent.
For the next few days, J.D.'s condition and treatment did not change much. His hemodynamic readings improved: cardiac index 2.7; PAP 45/18 mm Hg, with a mean of 26 mm Hg; pulmonary capillary wedge pressure 11 mm Hg; right atrial pressure 9 mm Hg; and heart rate 60/min. Changes in the settings of his pacemaker, such as shortened atrioventricular delay, were tried, but no improvement in LVOT obstruction occurred. Decreasing the pacemaker-initiated, atrioventricular conduction time changes the ventricular activation sequence, and for some patients this change may decrease LVOT obstruction. J.D. was kept sedated to optimize his comfort, and he was paralyzed to minimize oxygen demand while he was receiving mechanical ventilation. Continuous renal replacement therapy was started when anuria developed, and the serum creatinine level increased to 661 [micro]mol/L. Enteral tube feeding was started.
Five days after J.D.'s most recent acute deterioration, his condition had sufficiently improved, and treatment with phenylephrine, nitric oxide, and intravenous pancuronium and PAP monitoring were discontinued. His blood pressure increased to 185/90 mm Hg. DisopyramiAe phosphate was used to reduce blood pressure and keep his heart rate at less than 60/min, thereby reducing systolic pressure and LVOT obstruction. A chest radiograph revealed a large pleural effusion, and a chest tube was inserted. J.D. continued to require monitoring of electrolytes because of the acute renal failure.
Six days later, mechanical ventilation was discontinued, and J.D. was extubated. His urine output was greater than 30 mL/h, and the serum creatinine level stabilized at 124 [micro]mol/L. His electrolyte values had returned to normal. Continuous renal replacement therapy was discontinued, and the Foley catheter was removed. An echocardiogram showed mild hypokinesis of the posterior ventricular wall, isolated hypertrophy and sigmoid shape of the basal septum, and no gradient through the LVOT. J.D. was transferred to a general cardiac unit 3 weeks after insertion of his pacemaker. He required no further treatment for HOCM and was discharged to home 7 days later.
Pathophysiology of HOCM
HOCM is a genetic disease of the sarcomere, the contractile element of the cardiac muscle. Symmetrical or asymmetrical thickening of the interventricular septum and left ventricular free wall results in a left ventricular cavity that is normal or reduced in size, impedance to systolic outflow, and impairment of diastolic filling. (2,3) The mitral valve touches the thickened interventricular septum and blocks outflow (see Figure).
[FIGURE OMITTED]
Several mechanisms may be responsible for the impedance to left ventricular outflow in patients with HOCM. (4) A hyperdynamic left ventricle generates a high speed of flow through a narrowed outflow tract. This situation results in a high-speed ejection of blood, pulling the anterior leaflet of the mitral valve toward and onto the thickened septum; this movement is termed systolic anterior motion. (3) In the Venturi effect, the anterior mitral leaflet is sucked into the outflow tract as a result of the high-speed ejection.
A second mechanism that contributes to or may be responsible for the impedance of systolic outflow in patients with HOCM is related to displacement of the papillary muscles and an anterior shift and elongation of the mitral leaflets. Because of its anterior positioning and increased length, the leaflet may be positioned in the path of the outflow tract and propelled forward into the aortic outflow tract during ventricular ejection, causing a drag effect and enhancing outflow obstruction. (5,6)
LVOT obstruction is not fixed, rather it is always changing. Markedly increased systolic anterior motion and prolonged contact of the mitral valve leaflet with the septum result in an increased left ventricular outflow pressure gradient and mitral regurgitation. Increased pressure gradients and hypercontractility lead to a mismatch in oxygen supply and demand. Primary complications of outflow obstruction are reduced cardiac output and the development of congestive heart failure. Congestive heart failure results not only from outflow obstruction, which leads to backward failure as blood backs up from the left ventricle into the left atrium and lungs, but also from left ventricular diastolic dysfunction. Left ventricular diastolic dysfunction is due to impaired ventricular relaxation and filling of a hypertrophic and noncompliant left ventricle. (7)
Because of the complex pathophysiology, the precise mechanisms that cause the signs and symptoms of HOCM are not entirely known or understood. (7) Decreased cerebral perfusion may cause impaired consciousness and syncope. Syncope may also occur as a consequence of dysrhythmias. Pulmonary congestion may produce symptoms such as dyspnea, fatigue, and orthopnea. In outflow obstruction, a paradoxical split [S.sub.2] may be heard because of the delayed ejection of blood from the left ventricle as the pulmonic valve closes before the aortic valve. An [S.sub.4] may be heard as a consequence of a hypertrophic and noncompliant left ventricle. A narrowed LVOT is often accompanied by some mitral regurgitation, which may cause a systolic ejection murmur. (2)
Angina may be due to a mismatch in oxygen supply and demand, but it may also be due to "small vessel disease," which is common in patients with HOCM. (7) The small vessels are abnormal intramural coronary arteries characterized by thickened walls (primarily from increased amounts of intimal and medial collagen) and narrowed lumens. (7) Supraventricular dysrhythmias such as atrial fibrillation due to atrial enlargement are common in patients with HOCM. Patients often experience dysrhythmias as palpitations. Sudden cardiac death due to ventricular dysrhythmias is a major risk for some patients with HOCM. (2)
Discussion
J.D.'s underlying hypertrophic cardiomyopathy progressed to the obstructive subvariety when cardiac tamponade occurred after insertion of a pacemaker. The development of HOCM led to a cascade of complications, including angina, cardiogenic shock, and renal failure. As occurred in J.D., cardiac tamponade can develop when the pacemaker catheter migrates through the ventricular wall, creating a conduit for blood to leak into the pericardium. Acute tamponade after insertion of a pacemaker may cause rapid deterioration by restricting ventricular function, limiting contractility, and severely reducing cardiac output. In J.D., a slow leak may have occurred immediately after insertion of the pacemaker, causing sharp chest discomfort without decompensation.
Heparin infusion, which was started to inhibit thrombus formation, may have contributed to continued leakage into the pericardium. Heparin was discontinued as soon as tamponade was suspected, and protamine was administered to limit the bleeding. The nurses caring for J.D. were cognizant of the potential for tamponade and monitored him for distant heart sounds, pulsus paradoxus, electrical alternans, low-voltage complexes on electrocardiograms, ST-segment changes, hypotension, pulmonary congestion, elevated jugular venous distention, anxiety, fatigue, decreased level of consciousness, and decrease in hemoglobin level. Initially, J.D. had limited indications of tamponade, but some pulmonary congestion, anxiety, and fatigue. On the second day after the pacemaker surgery, distant heart sounds, pulsus paradoxus greater than 10 mm Hg, elevated jugular venous distention, and hypotension developed.
Normally, when cardiac tamponade is relieved by pericardiocentesis, the patient's status improves dramatically. J.D.'s condition improved temporarily after pericardiocentesis but deteriorated markedly again the next day, when cardiogenic shock was diagnosed. After pericardiocentesis, monitoring for recurrence of tamponade is needed. Although the restrictive component of tamponade contributed to the development of cardiogenic shock, a major factor in J.D.'s hemodynamic decompensation was the obstructed left ventricular outflow from the left ventricle, the key manifestation of HOCM.
Critical care nurses must understand that treatment of cardiogenic shock in a patient with LVOT obstruction differs from the traditional treatment of cardiogenic shock related to acute coronary syndromes. One goal in managing patients with LVOT obstruction and cardiogenic shock is to relieve the obstruction and thus improve hemodynamic status. The ideal hemodynamic status is one in which the patient is normotensive and asymptomatic.
Medications used to decrease LVOT obstruction are [beta]-blockers, [[alpha].sub.1]-agonists, calcium channel blockers, and disopyramide. (6,8) [beta]-Blockers, calcium channel blockers, and disopyramide have negative inotropic effects. The decrease in the force of ventricular contraction and ventricular ejection acceleration reduces systolic anterior motion of the mitral valve, aortic outflow obstruction, and the final aortic pressure gradient. (9) Another benefit of [beta]-blockers, calcium channel blockers, and disopyramide is their effect in decreasing heart rate (7); the decrease can increase ventricular preload by facilitating greater ventricular relaxation and longer filling before ventricular ejection. If treatment with [beta]-blockers and calcium channel blockers such as verapamil does not reduce pulmonary congestion, judicious use of diuretics is recommended. (7) [[alpha].sub.1]-Agonists increase the size of the functional outflow tract and decrease the LVOT pressure gradient by increasing systemic vascular resistance and end-systolic and end-diastolic left ventricular volume. (6) Another important difference in treating cardiogenic shock in patients with LVOT obstruction is avoiding treatment, such as use of vasodilators, intra-aortic balloon pumps, and inotropic agents, that aggravates the magnitude of the obstructive gradient. (6)
The medications used to treat LVOT obstruction in J.D. included [3-blockers (metoprolol and esmolol), a calcium channel blocker (verapamil), and an antiarrhythmic agent (disopyramide). Small doses of a diuretic (furosemide) were used to treat pulmonary congestion and the decrease in urine output. Dopamine was used in an attempt to increase urine output, but use of this drug at doses that would produce detrimental inotropic effects is not recommended. When disseminated intravascular coagulopathy and hypotension developed, intravenous fluid was administered cautiously to maintain adequate preload and afterload and prevent increased pulmonary congestion.
Norepinephrine and phenylephrine were used to increase blood pressure. These [[alpha].sub.1]-agonists have vasopressor effects and may have some dose-related cardiac [[beta].sub.1]-adrenergic effects; they should be used at low dosages to avoid inotropic effects, which could increase LVOT obstruction. (10) J.D. was monitored for other side effects of [[alpha].sub.1]-agonists, such as increased heart rate and vasoconstriction of renal, coronary, and pulmonary arteries. These side effects were of particular concern because he was being treated for acute renal failure, ischemic heart disease, pulmonary congestion, and compensatory sinus tachycardia.
The effects of all these medications must be monitored. The beneficial effects of negative inotropic agents ([beta]-blockers) in treatment of LVOT obstruction must be balanced against possible excessive depression of ventricular contractility or excessive effect on the peripheral vasculature, which could add to deterioration in hemodynamic status, pulmonary congestion, and/or renal failure.
Although nitroglycerin was used initially to treat angina in J.D., it was not resumed during the treatment of cardiogenic shock. Fortunately, J.D. did not have angina after the first day. His angina may have been controlled by the administration of [beta]-blockers, which reduced myocardial workload and oxygen demand.
Inhalation of nitric oxide via mechanical ventilation was established because of a high PAP of 50/25 mm Hg (mean, 30 mm Hg) and a pulmonary vascular resistance index of 625. Administering nitric oxide via mechanical ventilation allows selective dilatation of the pulmonary vessels; therefore, treatment with this agent reduced pulmonary pressures and right ventricular afterload without reducing left ventricular afterload. In patients with severe HOCM, PAPs are elevated because of backward failure due to obstructed forward flow of blood from the left ventricle. With high pulmonary pressures, the right ventricular pressures tend to increase, causing the interventricular septum to shift to the left. This leftward shift of the septum causes further narrowing of the LVOT. Nitric oxide was used to reduce pulmonary pressures, correcting the leftward septal shift and reducing LVOT obstruction.
Throughout J.D.'s illness, he and his family received strong emotional and educational support. Anxiety and fear were a consequence of the unexpected cascade of complications, particularly because his father and uncles had died of cardiac disease. To encourage the confidence of patients and patients' families, nurses and other healthcare professionals must be knowledgeable about assessments, monitoring, and interventions. Nurses must be vigilant in collecting a thorough history, consider the implications of that history, and do physical assessments even after routine low-risk procedures such as insertion of a pacemaker. After insertion of J.D.'s pacemaker, cardiogenic shock associated with cardiac tamponade and LVOT obstruction developed after the ventricle was perforated with a pacemaker lead. J.D.'s recovery depended on nurses' understanding the pathophysiology of HOCM and individualizing his treatment plan. Nurses play a pivotal role in assessment, detection of complications, monitoring, and interventions in patients with HOCM.
REFERENCES
(1.) Opie LH. The Heart: Physiology, From the Cell to Circulation. 3rd ed. Philadelphia, Pa: Lippincott Raven; 1998.
(2.) Oakley C. Aetiology, diagnosis, investigation, and management of the cardiomyopathies. BMJ. 1997;315:1520-1524.
(3.) Futterman LG, Lemberg L. New indications for dual chamber pacing: hypertrophic and dilated cardiomyopathy. Am J Crit Care. 1995;4:82-87.
(4.) Nakatani S, Marwick TH, Lever HM, Thomas JD. Resting echocardiographic features of latent left ventricular outflow obstruction in hypertrophic cardiomyopathy. Am J Cardiol. 1996;78:662-667.
(5.) Sherrid MV, Gunsburg DZ, Moldenhauer S, Pearle G. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2000;36:1344-1354.
(6.) Haley JH, Sinak LJ, Tajik AJ, Ommen SR, Oh JK. Dynamic left ventricular outflow tract obstruction in acute coronary syndromes: an important cause of new systolic murmur and cardiogenic shock. Mayo Clin Proc. 1999;74:901-906.
(7.) Maron BJ. Hypertrophic cardiomyopathy. Lancet. 1997;350:127-133.
(8.) Kajimoto K, Harada T, Imamura K, et al. Efficacy of continuous intravenous drip infusion of disopyramide in hypertrophic obstructive cardiomyopathy during cardiogenic shock: a case report [in Japanese]. J Cardiol. 2000;35:197-203.
(9.) Sherrid MV, Pearle G, Gunsburg DZ. Mechanism of benefit of negative inotropes in obstructive hypertrophic cardiomyopathy. Circulation. 1998;97:41-47.
(10.) Heerdt PM, Forstat RM. Heart rate control. In: Chernow B, ed. Essentials of Critical Care Pharmacology. 2nd ed. Baltimore, Md: Williams & Wilkins; 1994:297-312.
Anna Barkman, RN, MN, and Judy McCay, RN, BN. From Health and Community Studies, Mount Royal College (AB), and Coronary Intensive Care Unit, Foothills Medical Centre (JM), Calgary, Alberta, Canada.
COPYRIGHT 2002 American Association of Critical-Care Nurses
COPYRIGHT 2003 Gale Group