Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy, or HCM, is a disease of the myocardium (the muscle of the heart) in which a portion of the myocardium is hypertrophied (thickened) without any obvious cause.1 It had frequently been cited as one of the leading causes of sudden cardiac death in young athletes in the United States.2 However, this same work of passive surveillance saw exclusion of 52% of cases because they had structurally normal hearts drawing much skepticism to the previous hype. more...
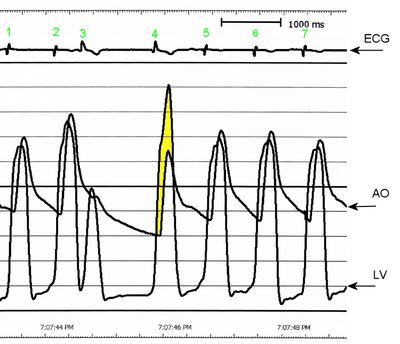
A cardiomyopathy is any disease that primarily affects the muscle of the heart. In HCM, the normal alignment of muscle cells is disrupted, a phenomenon known as myocardial disarray. HCM also causes disruptions of the electrical functions of the heart. HCM is believed to be due to a mutation in one of many genes that results in a mutated myosin heavy chain, one of the components of the myocyte (the muscle cell of the heart). Depending on the degree of obstruction of the outflow of blood from the left ventricle of the heart, HCM can be defined as obstructive or non-obstructive.
HCM is also known as idiopathic hypertrophic subaortic stenosis (IHSS) and hypertrophic obstructive cardiomyopathy (HOCM). A non-obstructive variant of HCM is apical hypertrophic cardiomyopathy 3, which is also known as nonobstructive hypertrophic cardiomyopathy and Japanese variant hypertrophic cardiomyopathy (since the first cases described were all in individuals of Japanese descent).
While most literature so far focuses on European, American, and Japanese populations, HCM appears in all racial groups. The incidence of HCM is about 0.2% to 0.5% of the general population.
Genetics
Hypertrophic cardiomyopathy is attributed to mutation in one of a number of genes that encode for one of the sarcomere proteins (usually effecting either the α or β myosin heavy chain on chromosome 14 q11.2-3). While the severity of the disease process is dependent on the particular gene mutation, about 80% of cases are inherited in an autosomal dominant pattern. Other gene mutations that are associated with HCM include mutations in α-tropomyosin (on chromosome 15), troponin T (on chromosome 1), and myosin-binding protein C (on chromosome 11). The prognosis is variable, based on the gene mutation.
The MYH7 gene (encoding the Β-myosin heavy chain) was the first specific gene identified in familial hypertrophic cardiomyopathy. About 50 percent of all familial cases involve mutation in the MYH7 gene. In individuals without a family history of HCM, the most common cause of the disease is also mutations of the gene that produces the β-myosin heavy chain. Many different mutations in this gene have been identified, and the prognosis is dependant on the particular mutation.
An insertion/deletion polymorphism in the gene encoding for angiotensin converting enzyme (ACE) has been associated with some cases of HCM. The D/D (deletion/deletion) genotype of ACE is associated with more marked hypertrophy of the left ventricle and may be associated with higher risk of adverse outcomes.8,9
Anatomic characteristics
Individuals with HCM have some degree of left ventricular hypertrophy. Usually this is an asymmetric hypertrophy, involving the inter-ventricular septum, and is known as asymmetric septal hypertrophy (ASH). This is in contrast to the concentric hypertrophy seen in aortic stenosis or hypertension. About 2/3 of individuals with HCM have asymmetric septal hypertrophy.
Read more at Wikipedia.org