* Context.-Previous investigators have reported discrepancies between hematologic, marrow morphologic, and cytogenetic responses to imatinib mesylate among patients with chronic myeloid leukemia (CML). In addition to disease refractoriness, rare instances of disease progression from chronic phase to blast crisis during imatinib therapy have recently been anecdotally reported.
Objectives.-To describe the clinicopathologic features of 3 patients with CML who rapidly progressed from chronic phase to blast crisis while taking imatinib and to perform a review of the literature.
Design.-Morphologic, immunophenotypic, and cytogenetic analyses were performed on the 3 patients at the time of initial diagnosis, during imatinib therapy, and at blast crisis.
Results.-The 3 patients were men, aged 39, 42, and 43 years. Two had been treated with hydroxyurea for 16 and 21 months before imatinib therapy, while 1 was started on a regimen of imatinib following diagnosis. Despite a hematologic response in all 3 patients, none of them achieved cytogenetic remission, and all progressed to blast crisis at 7 to 10 months of imatinib therapy. Blood findings during blast transformation were heterogeneous, including normal blood morphologic findings in 1 patient, leukocytosis with circulating blasts and basophilia in 1, and marked pancytopenia in 1. All 3 marrow specimens demonstrated moderate to marked diffuse reticulin fibrosis with more than 20% blasts. Clonal cytogenetic evolution was evident in 2 of the 3 patients and included an extra Philadelphia chromosome in both. All 3 patients underwent allogeneic bone marrow transplantation. One was alive with no evidence of disease at 14 month follow-up, while 2 had residual disease after bone marrow transplantation and died of complications at 4 and 5 months after transplantation.
Conclusions.-Blood data did not always reflect marrow status. Therefore, bone marrow follow-up is critical for monitoring of response. Our findings suggest that significant progression of marrow reticulin fibrosis during imatinib therapy can be an indicator for a return or progression of CML and, in some patients with CML, imatinib may promote cytogenetic clonal evolution, resulting in a poor response to treatment.
(Arch Pathol Lab Med. 2004;128:980-985)
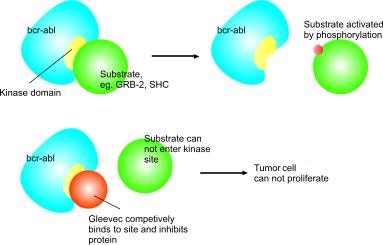
Chronic myeloid leukemia (CML) is a myeloproliferative disorder characterized by a reciprocal translocation between chromosomes 9 and 22. At the molecular level, the resultant fusion gene encodes a constitutively active Bcr-Abl tyrosine kinase, which has been shown to be necessary and sufficient to induce CML.12 Understanding of the molecular pathogenesis of CML led to Bcr-Abl targeted therapy; a selective Bcr-Abl tyrosine kinase inhibitor, imatinib mesylate (STI571, Gleevec; Novartis, East Hanover, NJ), was subsequently developed. Despite a high efficacy of imatinib in the treatment of CML, previous investigators have reported discrepancies between hematologie, marrow morphologic, and cytogenetic responses to imatinib.34 Imatinib induces complete hematologic remission following 1 to 2 months of drug administration, while complete morphologic marrow response may take 8 to 11 months. The cytogenetic response is variable and is low among patients in accelerated phase or blast crisis.4 Those patients with more advanced disease typically achieve transient hematologic remission followed by relapse, despite continued drug treatment. In addition to disease refractoriness, rare instances of disease progression from chronic phase to blast crisis during imatinib therapy have recently been anecdotally reported." Herein, we describe our experience with 3 such patients.
REPORT OF CASES
Case 1
A 39-year-old man presented in August 1999 for a routine physical examination and was found to have a white blood cell (WBC) count of 267000 cells/µL. Evaluation of the blood and a bone marrow specimen revealed CML. He was initially treated with hydroxyurea. Although his peripheral leukocyte count was normalized, the bone marrow continued to show morphologic features of chronic-phase CML. In May 2001, hydroxyurea was discontinued, and the patient was started on a regimen of imatinib at 400 mg/d. A follow-up bone marrow biopsy specimen in December 2001 revealed lymphoid blast crisis, although the patient was asymptomatic with normal complete blood counts. Cytogenetic studies showed the 9;22 translocation, without additional chromosome abnormalities. The patient failed induction chemotherapy with cytosine arabinoside (Ara-C) and idarubicin hydrochloride and was subsequently treated with prednisone, 5 cycles of vincristine sulfate, and high-dose Ara-C, followed by living-related donor bone marrow transplantation. The posttransplant bone marrow examinations demonstrated continued cytogenetic evidence of CML. Furthermore, the patient developed severe graft-versus-host disease and pulmonary cytomegalovirus infection. He died of respiratory failure in May 2002.
Case 2
A 42-year-old man was diagnosed as having CML in September 2000 after a routine laboratory examination revealed a WBC count of 17000 cells/µL. He was started on a regimen of imatinib at 400 mg/d and had a hematologic response. His 5-month bone marrow biopsy specimen revealed no definitive morphologic features of CML except for rare clusters of small megakaryocytes. However, cytogenetic studies demonstrated residual CML. In August 2001, examination of the blood showed leukocytosis with 27% circulating blasts. A bone marrow biopsy specimen confirmed myeloid blast crisis with clonal cytogenetic evolution by karyotypic analysis. He failed multiple courses of chemotherapy, including idarubicin and Ara-C, mitoxantrone hydrochloride and etoposide (VP-16), and high-dose Ara-C. He was then placed back on a regimen of imatinib at 600 mg/d, on which the blast count in marrow remained approximately 5%. In February 2002, the patient underwent a single cord blood stem cell transplantation that resulted in 100% engraftment. Two months later, his originally evolved clones recurred. In addition, he developed persistent fever of unknown origin and respiratory failure. With resuscitative efforts declined, the patient died in May 2002.
Case 3
A 43-year-old man presented in February 2000 to the hospital with abdominal fullness. He was found to have splenomegaly, with a WBC count of 193000 cells/µL. Examination of the blood and bone marrow revealed CML in chronic phase. He was initially placed on a regimen of hydroxyurea, but switched to imatinib (400 mg/d) in June 2001. He had a hematologic response to the treatment, but had failed to achieve cytogenetic remission when evaluated 5 months after initiation of imatinib. In March 2002, he was noted to be pancytopenic, with a hemoglobin level of 8.39 g/dL, leukocyte count of 2500 cells/µL, and platelets measuring 53000 cells/µL. Bone marrow examination revealed myeloid blast crisis with additional chromosome abnormalities. The patient received chemotherapy with combined mitoxantrone, vinorelbine tartrate, fludarabine phosphate, and dexamethasone sodium phosphate, but failed to achieve a complete remission. He underwent matched living-unrelated donor bone marrow transplantation in June 2002 and achieved 100% engraftment. He remained in remission at his last follow-up visit 14 months after blast crisis.
MATERIALS AND METHODS
Morphologic Findings
Wright-Giemsa-stained blood and bone marrow aspirate smears were examined by light microscopy. Core biopsy specimens were fixed in 37% buffered formaldehyde and B5 (1:9) fixative, embedded in paraffin, and processed routinely. The sections were stained with hematoxylin-eosin and examined under light microscopy.
Reticulin stain was performed on deparaffinized sections as described previously." The stained sections were examined under light microscopy, and the degree of reticulin fibrosis was graded as follows: 0, no increase of fibrosis; 1+, slight focal or diffuse fibrosis; 2+ , slight diffuse with moderate focal fibrosis; 3+ , moderate diffuse fibrosis; and 4+, marked diffuse fibrosis.
Immunohistochemistry for CD34 (dilution, 1:160; Signet Laboratories, Dedham, Mass) and terminal deoxynucleotidyl transferase (TdT) (dilution, 1:20; Dako, Carpinteria, Calif) was performed on 3-µm paraffin sections. For antigen retrieval, sections were placed in 200 mL of antigen retrieval citrate buffer (BioGenex, San Ramon, Calif), pH 6.8, and boiled for 5 minutes. After adding 50 mL of deionized water, the buffer was again brought to a boil for 5 minutes. The slides were allowed to cool in buffer for 20 minutes before further processing. The immunostaining was performed at room temperature on a TechMate automated immunostainer (Ventana Medical Systems, Tucson, Ariz) with a streptavidin-biotin-peroxidase detection system (Signet).
Flow Cytometry
Fresh bone marrow aspirate was lysed with ammonium chloride and washed in calcium- and magnesium-free phosphatebuffered saline (CELOX, Minneapolis, Minn). Cell suspensions were stained with a panel of monoclonal antibodies directly conjugated with fluorescein isothiocyanate, phycoerythrin, or peridinin chlorophyll protein at room temperature in the dark for 15 minutes. To detect nuclear TdT, cells were processed in fixative and permeabilization solutions (Caltag Laboratories, Burlingame, Calif) before incubation with TdT and CD45. Isotypic control (Simultest; Becton Dickinson, San Jose, Calif) was used as a negative control for TdT staining. All samples were analyzed using a 3color combination of antibodies on FACSCalibur with CellQuest software (Becton Dickinson). The antibodies against CD7, CD13, CD14, CD15, CD33, CD34, CD38, CD56, CDl 17, and HLA-DR were purchased from Becton Dickinson. Anti-CD36 was obtained from Pharmingen (San Diego, Calif), and anti-TdT was obtained from Supertechs (Bethesda, Md).
Blasts were gated using CD45 side scatterplots. An antigen was considered positively expressed when at least 20% of the gated cells expressed that antigen.
Cytogenetics
Conventional G-banded cytogenetic analysis was performed on bone marrow cells after 24- or 48-hour culture without mitogen stimulation. At least 20 metaphase cells were analyzed in each case, when possible. Fluorescence in situ hybridization (FISH) was performed using a translocation probe consisting of different colored, directly labeled BCR and ABL probes (Vysis, Downers Grove, III).
PATHOLOGIC FINDINGS
The patient characteristics are summarized in Table 1. At diagnosis, cytogenetic analysis by karyotyping revealed t(9;22)(q34;qll.2) as a sole chromosome abnormality in all 3 patients. The peripheral blood and bone marrow findings at the time of blast crisis are described in detail in this section and are summarized in Table 2.
Patient 1 had a normal complete blood cell count and blood smear morphologic findings at the time of blast crisis. His bone marrow aspirate was heavily hemodiluted. However, touch imprints of a trephine biopsy specimen revealed approximately 20% blasts with lymphoblastic morphologic features, including small- to medium-sized cells, homogeneous chromatin, inconspicuous nucleoli, and very scant cytoplasm. The marrow as seen on trephine biopsy was normocellular, with interstitial infiltrate by immature cells in small aggregates (Figure 1, A). Immunohistochemistry for CD34 (Figure 1, B) and TdT (Figure 1, C) confirmed that the immature cells were blasts, comprising 30% of the marrow cellularity. A reticulin stain of the marrow biopsy section showed moderate diffuse fibrosis. Limited immunophenotyping on cell suspension from a disaggregated trephine biopsy specimen demonstrated that blasts were lymphoid and positive for CD19, HLA-DR, and TdT, while negative for CD3, CD33, CD45, and CDl 17. No secondary cytogenetic abnormalities in addition to t(9;22)(q34;q11.2) were identified (Table 3).
Patient 2 had a bone marrow biopsy performed at 5 months of imatinib therapy, which showed slightly hypocellular marrow with a normal myeloid-erythroid ratio. The megakaryocytes were normal in number, but exhibited atypical small hypolobate morphologic features, with rare small clustering. Continued presence of a Philadelphia (Ph) chromosome with a BCR/ABL gene fusion was demonstrated by karyotyping and FISH analysis. At 10 months of therapy, he was found to have mild leukocytosis with basophilia and circulating blasts in the blood examination (Figure 2, A). The marrow was 100% cellular, with complete replacement by sheets of blasts (Figure 2, B). There was slight diffuse with moderate focal reticulin fibrosis. His blasts expressed CD7, CD13, CD33, CD34, CD38, CD117, HLA-DR, and TdT, but lacked other lymphoid markers, consistent with a myeloblast phenotype. Two related Ph chromosome-positive clones were detected in the marrow sample; 1 contained an extra Ph copy (Table 3).
Patient 3 showed a lack of cytogenetic response to imatinib at 5 months; by karyotyping and FISH analysis, a Ph chromosome as the sole chromosome abnormality with a BCR/ABL gene fusion was identified. He presented with marked pancytopenia at the time of blast crisis. Rare circulating blasts were identified on review of a blood smear. Flow cytometric immunophenotyping performed on the blood demonstrated a population of myeloblasts, which were positive for CD13, CD15, CD33, CD34, CD117, and HLA-DR, while negative for lymphoid markers. The bone marrow biopsy specimen showed 100% cellular marrow, with diffuse infiltrate by blasts and marked diffuse fibrosis with reticulin fibers surrounding individual blasts (Figure 3). Clones with additional chromosomal abnormalities were identified, including an extra Ph copy (Table 3).
COMMENT
Imatinib is a promising new therapeutic strategy in patients with CML. It produced a complete hematologic response in 88% and a cytogenetic response in 49% of patients in chronic phase and whose disease has failed to respond to Interferon alfa.7 However, the response in patients with advanced disease is poor; approximately 30% of patients in accelerated phase and 70% of patients in blast crisis failed to achieve hématologie remission.8,9 The most commonly identified mechanism for relapse is mutations within the Bcr-Abl kinase domain,10 which may lead to disrupted function of BCR-ABL or impaired imatinib binding. BCR-ABL amplification has also been seen in a subset of relapsed patients, including genomic amplification of BCR-ABL and overexpression of BCR-ABL transcripts.10 Acquisition of additional genetic abnormalities is detected in some patients with primary and acquired drug resistance.11,12
In this report, we describe 3 patients with progression from chronic phase to blast crisis during treatment with imatinib. A hematologic remission was attained in all 3 patients 1 to 2 months after starting imatinib, a timeline that is typical of other patients early in imatinib therapy as reported in the literature.3,5 In 1 patient, marrow morphologic examination at 5 months revealed a decrease in marrow cellularity and a normalized myeloid-erythroid ratio and megakaryocyte number, but atypical hypolobate megakaryocytes with rare focal clustering were observed. Cytogenetic analysis of the marrow at 5 months confirmed continued presence of the Ph chromosome. The abnormal morphologic finding of megakaryocytes was the only manifestation of residual CML in this patient. Although it alone is insufficient for a definitive diagnosis of residual CML, its presence should be cautiously interpreted in relation to other cytogenetic data and may warrant a close follow-up.
Patients treated with imatinib may develop cytopenia due to drug-related toxicity. Prater et al5 found a decrease in leukocyte count for 6 months from the time of treatment commencement in all of their patients. When the disease relapses, WBC counts might be expected to rise. We studied blood morphologic findings in our 3 patients at the time of blast crisis and found a heterogeneous presentation. One patient showed a typical blood picture for CML transformation, with leukocytosis, neutrophilic left shift with increased blasts, and basophilia. In contrast, another patient exhibited a normal blood smear, and the third had a blood smear with pancytopenia. Demonstration of blast crisis was possible only by an examination of the marrow. The time from imatinib therapy commencement to blast crisis was 7 to 10 months among our patients. These findings may indicate that careful bone marrow follow-up, particularly at early time points, is critical for monitoring of response.
Treatment with imatinib has been shown to result in significant regression of bone marrow fibrosis in CML patients.11 Marrow reticulin fibrosis in myeloproliferative disorders is thought to be due to abnormal secretion of transforming growth factor [beta] and platelet-derived growth factor by megakaryocytes.14 A decrease in marrow fibrosis in those treated patients can be explained by normalization of megakaryopoiesis by imatinib. Our 3 patients showed a significant increase in marrow reticulin fibrosis during the course of treatment: all had slight marrow reticulin fibrosis in the marrow biopsy specimens before imatinib treatment, but developed moderate to marked diffuse reticulin fibrosis at the time of blast transformation. Therefore, progression of marrow reticulin fibrosis during imatinib therapy could serve as an indicator for a return or progression of disease.
In one of our patients who presented with pancytopenia at the time of blast crisis, bone marrow revealed marked diffuse fibrosis, with reticulin fibers surrounding individual blasts in areas. The prominent fibrosis likely prevents blasts from circulating into peripheral blood, contributing to a pancytopenic blood picture. In another patient, the marrow aspirate was markedly hemodiluted because of marrow fibrosis and was inadequate for examination. However, the marrow biopsy sections demonstrated scattered interstitial immature cells, and immunohistochemical studies for CD34 and TdT confirmed the diagnosis of blast crisis. In the case of a dry aspiration, careful examination for blasts in the core biopsy specimen is critical for diagnosis of CML transformation and may require CD34 immunohistochemistry. Analysis with an expanded panel, including CD117 (for myeloblasts), TdT (for lymphoblasts), and glycophorin-A (for erythroblasts), may be helpful in some cases.
Clonal cytogenetic evolution was detected during imatinib therapy in 2 of our 3 patients. Both patients acquired an extra Ph chromosome in addition to other secondary chromosome abnormalities. The duration of chronic phase in these 2 patients was 1 to 2 years, whereas chronic phase generally lasts 3 to 5 years before disease progression. A sole Ph chromosome with a BCR/ABL gene fusion was confirmed by karyotyping and FISH analysis at 5 months of imatinib therapy in both patients, suggesting that the extra Ph chromosome identified at blast crisis was acquired during treatment. It is notable that c-Abl can be activated by certain DNA-damaging agents and may contribute to the induction of programmed cell death. It interacts with several proteins in DNA repair processes. 15-17 The inhibitory effects of imatinib on c-Abl may increase genetic instability and favor development of cytogenetic clonal evolution. Cytogenetic clonal evolution present before imatinib therapy was not associated with a significant difference in cytogenetic response to imatinib therapy.18 In contrast, we found that our patients with newly developed chromosome abnormalities during imatinib therapy were resistant to higher-dose imatinib or cytotoxic chemotherapy. Our study suggests that therapeutic modalities beyond imatinib may be necessary for such patients.
All 3 of our patients failed to achieve a cytogenetic response. Failure to achieve cytogenetic response in the first 6 months of therapy has been shown by Branford et al19 to be associated with the presence of mutations: 19 (38.0%) of 50 patients without a major cytogenetic response had mutations, compared with 8 (8.5%) of 94 patients with a major cytogenetic response (P
In summary, we describe 3 patients with CML who progressed from chronic phase to blast crisis during imatinib therapy, all within 3 years from diagnosis. With imatinib as the current standard of care in the treatment of chronicphase CML, our understanding of the duration of the various phases of CML, including time to blast crisis, may need to be adjusted accordingly. Furthermore, the blood data at the time of transformation did not always reflect marrow status. We found that progression of marrow reticulin fibrosis during imatinib therapy was associated with disease progression, and that CD34 immunostaining of the core biopsy specimen was useful in diagnosing early blast transformation when marrow aspiration is unsuccessful because of reticulin fibrosis. Our findings suggest that imatinib therapy may promote development of additional cytogenetic abnormalities, resulting in a poor response to treatment. We anticipate that future larger studies and molecular studies will allow us to understand the prevalence of this phenomenon and its molecular basis.
References
1. Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:10791082.
2. Gishizky ML, Witte ON. Initiation of deregulated growth of multipotent progenitor cells by bcr-abl in vitro. Science. 1992;256:836-839.
3. Braziel RM, Launder TM, Druker B), et al. Hematopathologic and cytogenetic findings in imatinib mesylate-treated chronic myeiogenous leukemia patients: 14 months' experience. Blood. 2002;100:435-441.
4. Kantarjian HM, Cortes), O'Brien S, et al. Imatinib mesylate (STI571) therapy for Philadelphia chromosome-positive chronic myeiogenous leukemia in blast phase. Blood. 2002:99:3547-3553.
5. Prater JL, Tallman MS, Variakojis D, et al. Chronic myeloid leukemia following therapy with imatinib mesylate (Cleevec): bone marrow histopathology and correlation with genetic status. Am J Clin Pathol. 2003;119:833-841.
6. Nguyen PL. Collection, processing, and examination of bone marrow specimens. In: Jaffe ES, Harris NL, Vardiman JW, eds. Diagnostic Hematopathology. New York, NY: Harcourt Health Sciences. In press.
7. Cohen MH, Williams C, Johnson JR, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002:8:935-942.
8. Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928-1937.
9. Sawyers CL, Hochhaus A, Feldman E, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase Il study. Blood. 2002:99:3530-3539.
10. Corbin AS, La Rosee P, Stoffregen EP, Druker BJ, Deininger MW. Several Bcr-Abl kinase domain mutants associated with imatinib mesylate resistance remain sensitive to imatinib. Blood. 2003;101:4611-4614.
11. Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002; 1 6:2190-2196.
12. Hochhaus A, Kreil S, Corbin A, et al. Roots of clinical resistance to STI571 cancer therapy. Science. 2001:293:2163.
13. Beham-Schmid C, Apfelbeck U, Sill H, et al. Treatment of chronic myelogenous leukemia with the tyrosine kinase inhibitor STI571 results in marked regression of bone marrow fibrosis. Blood. 2002:99:381-383.
14. Yang M, Khachigian LM, Hicks C, Chesterman CN, Chong BH. Identification of PDGF receptors on human megakaryocytes and megakaryocytic cell lines. Thromb Haemost. 1997;78:892-896.
15. Chen C, Yuan SS, Liu W, et al. Radiation-induced assembly of Rad51 and Rad52 recombination complex requires ATM and c-Abl. J Biol Chem. 1999:274: 12748-12752.
16. Kharbanda S, Pandey P, Jin S, et al. Functional interaction between DNAPK and c-Abl in response to DNA damage. Nature. 1997:386:732-735.
17. Yuan ZM, Shioya H, Ishiko T, et al. p73 Is regulated by tyrosine kinase cAbl in the apoptotic response to DNA damage. Nature. 1999:399:814-817. 18. Cortes JE, Talpaz M, Giles F, et al. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood. 2003:101:3794-3800.
19. Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003:102:276-283.
20. Kantarjian HM, Cortes JE, O'Brien S, et al. Imatinib mesylate therapy in newly diagnosed patients with Philadelphia chromosome-positive chronic myelogenous leukemia: high incidence of early complete and major cytogenetic responses. Blood. 2003:101:97-100.
Yin Xu, MD, PhD; Andrea E. Wahner, MS; Phuong L. Nguyen, MD
Accepted for publication May 7, 2004.
From the Department of Pathology and Laboratory Medicine, University of Minnesota School of Medicine, Minneapolis.
The abstract was presented as a poster during the Annual Meeting of the United States and Canadian Academy of Pathology, Vancouver, British Columbia, March 9, 2004.
The authors have no relevant financial interest in the products or companies described in this article.
Reprints: Yin Xu, MD, PhD, Department of Pathology, The University of Texas Southwestern Medical Center at Dallas, 5323 Harry Nines Blvd, Dallas, TX 75390-9073 (e-mail: yin.xu@utsouthwestern.edu).
Copyright College of American Pathologists Sep 2004
Provided by ProQuest Information and Learning Company. All rights Reserved