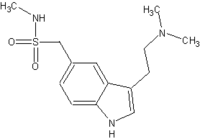
Imitrex
Sumatriptan (Imitrex®, Imigran®) is a triptan drug originally developed by Glaxo for the treatment of migraine headaches. Several dosage forms for sumatriptan have been approved, including tablets, solution for injection, and nasal inhalers. Sumatriptan was the first triptan available (in 1993), and is available only by prescription in the United States. more...
Mode of action
Sumatriptan is a 5-HT (5-HT1D) agonist. The specific receptor subtype it activates is present in the cranial and basilar arteries. Activation of these receptors causes vasoconstriction of those dilated arteries. Sumatriptan is also shown to decrease the activity of the trigeminal nerve.
Pharmacokinetics
Sumatriptan is administered in several forms; tablets, subcutaneous injection, and nasal spray. Oral administration (as succinate) suffers from poor bioavailability, partly due to presystemic metabolism — some of it gets broken down in the stomach and bloodstream before it reaches the target arteries. A new rapid-release tablet formulation has the same bioavailability, but the maximum concentration is achieved on average 10-15 minutes earlier. When injected, sumatriptan is faster acting (usually within a minute), but the effect lasts for a shorter time. Sumatriptan is metabolised primarily by monoamine oxidase A into an indole acetic acid analogue, part of which is further conjugated with glucuronic acid. These metabolites are excreted in the urine and bile.
Read more at Wikipedia.org