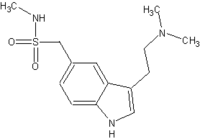
People who suffer the acute pain of migraine headaches may find relief with a new injectable drug FDA approved Dec. 28. 1992, called Imitrex (sumatriptan).
In two clinical trials in the United States involving more than 1,000 patients with acute migraine headaches, a 6-milligram injection of Imitrex relieved the pain in 75 percent of the patients within one hour. A few felt pain relief within 10 to 30 minutes, and 80 percent of the patients felt pain relief after two hours.
Although effective in alleviating pain in a high proportion of patients, Imitrex is not a cure for migraines. In addition, people with underlying heart disease should not take the drag because it may constrict coronary arteries.
"FDA advises, as a precautionary measure in people who might have underlying coronary artery disease, that physicians consider giving the first injection in their Office," says Carl Peck, M.D., director of FDA's Center for Drug Evaluation and Research. "This will allow patients to be observed and treated for adverse reactions, if necessary."
Peck said that having the first injection given by a doctor would also ensure proper use of the auto-injector. The device injects the maximum recommended dose of 6 milligrams under the skin.
Patients who need a lower dose will get a separate syringe and single-dose vial.
The drug's side effects include a mild, short-lived rise in blood pressure, fatigue, and drowsiness.
Educational information, including a videotape and patient brochure, on how to use the automatic injection device and the injectable vials will be provided to physicians and their patients.
The patient brochure advises women of childbearing age to consider carefully the benefits and risks of using Imitrex. In animal studies, fatal deaths occurred in rabbits, but not in rats, given the drug. As part of extensive post-marketing studies, the manufacturer will collect data on women who become pregnant while on the drag.
Imitrex is manufactured by Glaxo, Inc., Research Triangle Park, N.C.
(For more information on migraines and other headache pain, see "Migraine, Cluster and Tension: Headache Misery May Yield to Proper Treatment in the September 1992 FDA Consumer.)
COPYRIGHT 1993 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group