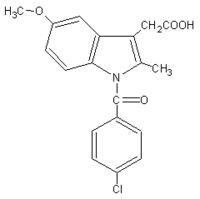
UV Erythema Reducing Capacity of Mizolastine Compared to Acetylsalicylic Acid or both Combined in Comparison to Indomethacin^(paragraph)
ABSTRACT
UV light exerts hazardous effects such as induction of skin cancer and premature skin aging. In this study we evaluated an assumptive anti-inflammatory effect of the nonsedative histamine H1-receptor antagonist, mizolastine, on UV-induced acute sunburn reaction. Therefore, a clinical, randomized, double-blind, four-arm, crossover study was conducted in healthy young female volunteers (skin type II) comparing the UV sensitivity under mizolastine, acetyl-salicylic acid (ASA), indomethacin or a mizolastine/ASA combination. Moreover, HaCaT keratinocytes were incubated with mizolastine under various UV treatment modalities in vitro to study its effect on the release of inflammatory cytokines, ie. interleukin (IL)-- 1alpha, IL-6 and tumor necrosis factor alpha (TNF-alpha). All three drugs were effective in suppressing the UVB-, UVA- and combined UVA/UVB-erythema. However, the strongest effects were observed using the combined treatment with both 250 mg ASA and 10 mg mizolastine. An inhibitory effect in vitro of 10 nM mizolastine upon UV-induced cytokine release from HaCaT keratinocytes was observed for IL-1alpha at 24 h after 10 J/cm^sup 2^ UVA1, for IL-6 at 48 h after 10 J/cm^sup 2^ UVA1 and 30 mJ/cm^sup 2^ UVB, and also for TNF-alpha at 4 h after 10 J/cm^sup 2^ UVA, 10 J/cm^sup 2^ UVA1 and 30 mJ/cm^sup 2^ UVB, respectively. The combination of mizolastine and ASA can be strongly recommended as a protective measure against UV erythema development with a lower unwanted side effect profile than that of the hitherto treatment modality, ie. indomethacin.
^^Abbreviations: ASA, acetyl-salicylic acid; CIELAB, Commission International de l'Eclairage; COX, cyclooxygenase; IL, interleukin; LO, lipoxygenase; MED, minimal erythema dose; NSAID, nonsteroidal antiphlogistic drugs; PMNL polymorphonuclear neutrophils; TNF-alpha, tumor necrosis factor alpha.
It is a well-known fact that UVB irradiation, in particular, leads to a visible erythema as local sign of inflammation by turning on the arachidonic acid cascade. There is strong evidence that prostaglandins, in particular, mainly of the E series are involved in this inflammatory process. Indomethacin applied systemically after an erythematogenic dose of UVB light suppresses the development of erythema by the inhibition of the prostaglandin synthesis cascade (40,41). In addition, the topical application of indomethacin prior to UV exposure in humans in vivo was shown to act as a potent UVB and UVA photoabsorption filter (23).
In the case where an unwanted situation of sunburn has occurred it is known that the course of development can be slowed down by administration of COX inhibitors during the first hours such as indometacin or high dose of ASA. Given the fact that leukotriens are also involved in the arachidonic cascade we raised the question if the combination of ASA plus an anti-inflammatory LO pathway inhibiting antihistamine of the mizolastine type could influence the course of erythema development. Knowing that I MED produces only a visible erythema and this is apparently not strong enough for the differentiation of the anti-inflammatory potency of drugs we used a higher UV dose of 2X MED, which significantly increased the inflammatory response.
The ASA- or indomethacin-dependent, in contrast to the mizolastine monotherapy-dependent, decrease of (delta)a on UVB-irradiated skin compared to control skin of the same individuum has to be related to the fact that the NSAID inhibit the COX pathway (41-43) but the latter drug has an inhibitory effect on the 5-LO pathway (19).
In our study ASA, indomethacin and mizolastine all together were able to decrease the inflammatory response in the UV ranges selected (UVB, UVA/UVB, UVA). Among these three given drugs indomethacin as a single agent showed no difference compared to mizolastine plus ASA in the UVA[UVB range. Our hypothesis that mizolastine in combination with ASA could be additive in its anti-inflammatory suppressive efficacy was confirmed by our results showing that 10 mg mizolastine plus 250 mg ASA was as effective or even more effective than indomethacin in the UVB range.
In order to contribute to the further clarification of the mode of action of mizolastine in preventing UV-induced inflammation we studied the drug's effect in an in vitro system using HaCaT keratinocytes as a model for the epidermal compartment which is the primary physical tissue target of any UV irradiation. Under the chosen conditions we could demonstrate that mizolastine in the nanomolar dose range was able to reduce substantially the release of IL-1alpha induced by UVA1 and the release of TNF-alpha induced by UVA, UVA1 or UVB, respectively. This indicates that the anti-inflammatory efficacy of mizolastine may be explained partly by a protection of the keratinocyte layer as the upper most living skin component against UV damage resulting in a diminished release of proinflammatory cytokines from the epidermis into the dermo-vascular micromilieu.
Taken together, the clinical in vivo and experimental in vitro results revealed that a highly significant suppression of UVB-induced erythema response by the combination of mizolastine plus ASA was most probably based on the additional inhibition of the leukotriene pathway accompanied by the prevention of an epidermal release of proinflammatory cytokines.
From our data we thus finally draw the conclusion that the combination of oral doses of 10 mg mizolastine and 250 mg ASA induces additive or synergistic UV-protective processes which might be important for future therapeutic recommendations to achieve an immediate suppression of the early phase of UV-induced erythema. This drug combination may be of interest for forthcoming studies seeking to develop pharmacological prevention strategies against chronic long-term UV skin damage.
Acknowledgements-HaCaT keratinocytes were kindly provided by Prof. Dr. N. E. Fusenig (German Cancer Research Center, Heidelberg, Germany). The technical assistance of Mrs. R. Adolf is gratefully appreciated.
(paragraph)Posted on the website on 10 August 2001.
^Some of the material was presented at the 30th Annual Meeting of the European Society for Dermatological Research, 21-23 September 2000, Berlin, Germany, and at the 9th Congress of the European Academy of Dermatology & Venereology, 11-15 October 2000, Geneva, Switzerland.
REFERENCES
1. Serraino, D., L. Fratino, W. Gianni, C. Campisi, M. Pietropaolo, G. Trimarco and V. Marigliano (1998) Epidemiological aspects of cutaneous malignant melanoma. (Review) Oncol. Rep. 5, 905-909.
2. Dennis, L. K. (1999) Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch. Dermatol. 135, 275-280.
3. Wikonkal, N. M. and D. E. Brash (1999) Ultraviolet radiation induced signature mutations in photocarcinogenesis. J. Investig. Dermatol. Symp. Proc. 4, 6-10.
4. Langley, R. G. and A. J. Sober (1997) A clinical review of the evidence for the role of ultraviolet radiation in the etiology of cutaneous melanoma. Cancer Investig. 15, 561-567.
5. Elwood, J. M. and J. Jopson (1997) Melanoma and sun exposure: an overview of published studies. Int. J. Cancer 73, 198203.
6. Podda, M., M. G. Traber, C. Weber, L. J. Yan and L. Packer (1998) UV irradiation depletes antioxidants and causes oxidative damage in a model of human skin. Free Radic. Biol. Med. 1 24(1), 55-65.
7. Biesalski, H. K., C. Hemmes, W. Hopfenmuller, C. Schmid and H. P. Gollnick (1996) Effects of controlled exposure of sunlight on plasma and skin levels on beta-carotene. Free Radic. Res. 24, 215-224.
8. Fitzpatrick, T. B., R. A. Johnson, M. K. Polano, D. Suurmond and K. Wolff (1994) Color Atlas and Synopsis of Clinical Dermatology. 2nd ed., 213 pp. McGraw-Hill, New York.
9. Benavides, J., H. Schoemaker, C. Dana, Y. Claustre, M. Delahaye, M. Prouteau and P. Manoury (1995) In vivo and in vitro interaction of the novel selective histamine Hi receptor antagonist mizolastine with Hi receptors in the rodent. Arzneimittelforschung 45, 551-558.
10. Slater, J. W., A. D. Zechnich and D. G. Haxby (1999) Secondgeneration antihistamines. A comparative review. Drugs 57, 3147.
11. Rosenzweig, P., H. Captain, S. Chaufour, N. Ulliac, M. J. Cabanis and 1. J. Thebault (1995) Comparative wheat and flare study of mizolastine versus terfenadine, cetirizine, loratadine and plazebo in healthy volunteers. Br. J. Clin. Pharmacol. 40, 459-465.
12. Michel, L., M. Murrieta-Aguttes, F. Jean-Louis, D. Levy and L. Dubertret (2000) Humoral and cellular responses to histamine
and pollen allergen in a skin chamber model: effect of mizolastine. Ann. Allergy Asthma Immunol. 85, 64-69.
13. Vargaftig, B. B. (1993) Mechanism of experimental bronchopulmonary hyperresponsiveness as related to eosinophils. In New developments in the Therapy of Allergic Disorders and Asthma (Edited by S. Z. Larger, M. K. Church, B. B. Vargaftig and S. Nicosia), Int. Acad. Biomed. Drug Res. 6, 27-32. Basel, Karger.
14. Goldhill, J., P. Pichat, N. Roome, I. Angel and S. Arbilla (1998) Effect of mizolastine on visceral sensory afferent sensitivity and inflammation during experimental colitis. Arzneimittelforschung 48, 179-184.
15. Wagner, J. G. and R. A. Roth (2000) Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol. Rev. 52, 349-374.
16. Horizoe, T., N. Nagakura, K. Chiba, H. Shirota, M. Shinoda, H. Numata, S. Kobayashi and C. Abe (1999) Effects of ER-34122, a novel dual 5-lipoxygenase/cyclooxygenase inhibitor, on indices of early articular lesion in MRL/MpJ-1pr/1pr mice. Inflamm. Res. 48, 432-436.
17. Horizoe, T., N. Nagakura, K. Chiba, H. Shirota, M. Shinoda, N. Kobayashi, H. Numata, Y. Okamoto and S. Kobayashi (1998) ER-34122, a novel dual 5-lipoxygenase/cyclooxygenase inhibitor with potent anti-inflammatory activity in an arachidonic acid-induced ear inflammation model. Inflamm. Res. 47, 375383.
18. Pichat, P., I. Angel and S. Arbilla (1998) Anti-inflammatory properties of mizolastine after oral administration on arachidonic acid-induced cutaneous reaction in the rat. Drug Res. 48, 173-178.
19. Sudo, K., K. Nagai and N. Yamada (1998) Inhibitory effect of mizolastine an 5-lipoxygenase. Jpn. Pharmacol. Ther. 26 (Suppl. 4), 155-157.
20. Abeyama, K., W. Eng, J. V. Jester, A. A. Vink, D. Edelbaum, C. J. Cockerell, P. R. Bergstresser and A. Takashima (2000) A role for NF-KB-dependent gene transactivation in sunburn. J. Clin. Investig. 105, 1751-1759.
21. Bickel, A., S. Dorfs, M. Schmelz, C. Forster, W. Uhl and H. 0. Handwerker (1998) Effects of antihyperalgesic drugs on experimentally induced hyperalgesia in man. Pain 76, 317-325.
22. Kuwamoto, K., H. Miyauchi-Hashimoto, K. Tanaka, N. Eguchi, T. Inui, Y. Urada and T. Horio (2000) Possible involvement of enhanced prostaglandin E2 production in the photosensitivity in xeroderma pigmentosum group A model mice. J. Investig. Dermatol. 114, 241-246.
23. Schwarz, T., F. Gschnait and F. Greiter (1985) Photoprotective effect of topical indomethacin-an experimental study. Dermatologica 171, 450-458.
24. Paunesku, T., C. M. Chang-Liu, P. Shearin-Jones, C. Watson, J. Milton, J. Oryhon, D. Salbergo, A. Milosavljevic and G. E. Woloschak (2000) Identification of genes regulated by UV/salicylic acid. Int. J. Radiat. Biol. 76, 189-198.
25. Hruza, L. L. and A. P. Pentland (1993) Mechanisms of UVinduced inflammation. J. Investig. Dermatol. 100, 35S-415. 26. Gasparro, F. P. (2000) Sunscreens, skin photobiology, and skin
cancer: the need for UVA protection and evaluation of efficacy. Environ. Health Perspect. 108 (Suppl.), 71-78.
27. Ullrich, S. E. and D. A. Schmitt (2000) The role of cytokines in UV-induced systemic immune suppression. J. Dermatol. Sci. 23 (Suppl. 1), SIO-512.
28. Hill, L. L., V. K. Shreedhar, M. L. Kripke and L. B. Owen-- Schaub (1999) A critical role for Fas ligand in the active suppression of systemic immune responses by ultraviolet radiation. J. Exp. Med. 189, 1285-1294.
29. Toth-Jakatics, R., S. Jimi, S. Takebayashi and N. Kawamoto (2000) Cutaneous malignant melanoma: correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum. Pathol, 31, 955-960.
30. de Gruijl, F. R. (1999) Skin cancer and solar UV radiation. Eur. J. Cancer 35, 2003-2009.
31. Norval, M., J. Garssen, H. Van Loveren and A. A. el-Ghorr (1999) UV-induced changes in the immune response to micro
bial infections in human subjects and animal models. J. Epidemol. 9 (Suppl.), S84-592.
32. Brink, N., M. Szamel, A. R. Young, K. P. Wittern and J. Bergemann (2000) Comparative quantification of IL-lbeta, IL-10, IL-lOr, TNF-alpha and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflamm. Res. 49, 290-296.
33. Starcher, B. (2000) Role for tumor necrosis factor-a receptors on ultraviolet induced skin tumours. Br. J. Dermatol. 142, 1440-1147.
34. Moehrle, M., W. Koehle, K. Dietz and G. Lischka (2000) Reduction of minimal erythema dose by sweating. Photodermatol. Photoimmunol. Photomed. 16(6), 260-262.
35. Weatherall, I. L. and B. D. Coombs (1992) Skin color measurements in terms of CIELAB color space values. J. Investig. Dermatol. 99, 468-473.
36. Robertson, A. R. (1977) The CIE 1976 color difference formula. Color Res. Appl. 2, 7-11.
37. Bonnekoh, B., B. Farkas, J. Geisel and G. Mahrle (1990) Lactate dehydrogenase release as an indicator of dithranol-induced membrane injury in cultured human keratinocytes-a time profile study. Arch. Dermatol. Res. 282, 325-329.
38. Gollnick, H. P. M., W. Hopfenmuller, C. Hemmes, S. C. Chun, K. Sundermeier and H. K. Biesalski (1996) Systemic beta carotene plus topical UV-sunscreen are an optimal protection against harmful effects of natural UV-sunlight: results of the Berlin-Eilath study. Eur. J. Dermatol. 6, 200-205.
39. Grundmann, J.-U. and H. P. M. Gollnick (1999) Prevention of ultraviolet ray damage: external and internal sunscreens. Ther. Umsch. 56, 225-232.
40. Hughes, G. S., S. F. Francom, L. K. Means, D. F. Bohan, C. Caruana and M. Holland (1992) Synergistic effects of oral nonsteroidal drugs and topical corticosteroids in the therapy of sunburn in humans. Dermatology 184, 54-58.
41. Snyder, D. K. and W. Eaglstein (1974) Topical indomethacin and sunburn. Br. J. Dermatol. 90, 91-93.
42. Fischer, S. M., H. H. Lo, G. B. Gordon, K. Seibert, G. Kelloff, R. A. Lubet and C. J. Conti (1999) Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol. Carcinog. 25, 231-240.
43. Livingston, A. (2000) Mechanism of action of nonsteroidal antiinflammatory drugs. Vet. Clin. N. Am. Small Anim. Pract. 30, 773-781.
Jens-Uwe Grundmann*, Raik Bockelmann, Bernd Bonnekoh and Harald P. M. Gollnick
Department of Dermatology and Venereology, Otto-von-Guericke-University, Magdeburg, Germany
Received 25 January 2001; accepted 6 July 2001
* To whom correspondence should be addressed at: Department of Dermatology and Venereology, Otto-von-Guericke-University, Leipziger Strasse 44, D-39120 Magdeburg, Germany. Fax: 49-391-67-15283; e-mail: jens-uwe.grundmann@medizin.uni-magdeburg.de
Copyright American Society of Photobiology Oct 2001
Provided by ProQuest Information and Learning Company. All rights Reserved