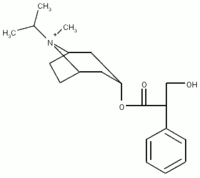
Study objective: Inhaled bronchodilators are the first-line pharmacotherapy against COPD. The purpose of the present study was to investigate the effects of [[beta].sub.2]-agonists and anticholinergic agents on the exercise capacity of patients with COPD.
Methods: A total of 67 stable patients with COPD were recruited at the Kyoto University Hospital. After inhaling 400 [micro]g salbutamol, 80 [micro]g ipratropium bromide, or an identical placebo in a randomized, double-blind, crossover fashion, the patients performed cycle endurance tests at a constant workload of 80% of the maximum work rate reached on progressive cycle ergometry, and the endurance time was recorded.
Results: Both salbutamol and ipratropium bromide significantly improved the endurance time by 29 s (15%; p < 0.001) and 27 s (14%; p < 0.001), respectively, in comparison with the placebo. However, there was no statistically significant difference between them (p = 0.71). The dyspnea ratios were also similarly reduced by both bronchodilators. The difference in the endurance time between therapy with salbutamol and placebo was significantly, but moderately, related to the difference between therapy with ipratropium bromide and placebo. In addition, there were no relationships, or only weakly significant relationships, between the change in FE[V.sub.1] and the change in the endurance time, the highest oxygen uptake, and the highest minute ventilation for both salbutamol and ipratropium bromide.
Conclusions: Therapy with both salbutamol and ipratropium bromide improved exercise capacity, as evaluated by the endurance time, and reduced dyspnea similarly in patients with COPD. In addition, the effects of the different bronchodilators on exercise capacity varied within individuals, and a complex mechanism may be responsible for the different effects of these two bronchodilators on exercise capacity vs airflow limitation. These results support the conclusion that both types of inhaled bronchodilators can be used as first-line drugs for the treatment of stable patients with COPD.
Key words: COPD; dyspnea; exercise endurance; ipratropium bromide; salbutamol
Abbreviations: DLCO = diffusing capacity of the lung for carbon monoxide; HR = heart rate; Sa[O.sub.2] = arterial oxygen saturation; VE = minute ventilation; VC[O.sub.2] = carbon trioxide output; V[O.sub.2] = oxygen uptake; Wmax = maximum work rate
**********
Inhaled bronchodilators such as [[beta].sub.2]-agonists and anticholinergic agents have different modes of action, and they have been used as the main pharmacotherapy for stable patients with COPD. Airway smooth muscle tone is maintained by a balance of activity between the sympathetic (adrenergic) and parasympathetic (cholinergic) autonomic nervous systems. (1) Bronchodilation may be obtained either by stimulating the adrenergic receptors with [[beta].sub.2]-agonists, or by inhibiting the action of acetylcholine at muscarinic receptors with anticholinergic agents. (1) In terms of the acute bronchodilating effects of [[beta].sub.2]-agonists and anticholinergic agents, both drugs have been reported to be similarly effective. (2-4) However, exercise capacity and dyspnea have recently become the major treatment end points in patients with COPD, as they are considered to be directly related to the patient's quality of life.
Although the effects of selective [[beta].sub.2]-agonists on exercise capacity are controversial in COPD patients, negative reports appear to be more prevalent. (5,6) Furthermore, the dose-dependent effects of [[beta].sub.2]-agonists have not been observed for exercise capacity, unlike those for FE[V.sub.1]. (7) On the other hand, with regard to anticholinergic agents, it has been previously demonstrated that their effects on exercise capacity in COPD patients depended on the dose administered (8) and the type of exercise performance tests that had been carried out. (9) In particular, we have reported (9) that a cycle endurance test was much more sensitive in detecting the effects of oxitropium bromide on exercise capacity than were the 6-min walking test and progressive cycle ergometry in patients with COPD. Therefore, when comparing the effects of [[beta].sub.2]-agonists and anticholinergic agents on exercise capacity, it is considered that the lower doses may not have a meaningful impact on the variables measured, and that a submaximal endurance test would be suitable for detecting the changes.
We hypothesized that both classes of bronchodilators would improve exercise capacity and reduce dyspnea in COPD patients. Therefore, we investigated whether there might be some differences between their effects. In the present study, we compared the effects of the adrenergic agent salbutamol and the anticholinergic agent ipratropium bromide on exercise capacity and dyspnea, using a cycle endurance test, in patients with COPD.
MATERIALS AND METHODS
Patients
We recruited a total of 67 consecutive patients with clinically stable COPD, as defined by the American Thoracic Society, (10) between January 1999 and December 2001. The entry criteria for the study included the following: (1) age > 50 years; (2) a history of cigarette smoking of > 20 pack-years; (3) FE[V.sub.1] of < 80% of the predicted value; and (4) best postbronchodilator FE[V.sub.1]/FVC ratio of < 0.7. The present study included those who had mild-to-severe airflow limitation. The exclusion criteria included the following: (1) an exacerbation of airflow limitation within the last 3 months; (2) a history of asthma; (3) the presence of other diseases likely to affect exercise; (4) severe hypoxemia (defined as a Pa[O.sub.2] of < 60 mm Hg at rest); and (5) treatment with oral bronchodilators, and oral or inhaled corticosteroids over the preceding 4 weeks. Written informed consent was obtained from all patients before the initiation of the study.
All patients underwent baseline pulmonary function testing at least 12 h after the withdrawal of their bronchodilator medications. According to the methods recommended by the American Thoracic Society, (11) the spirometric testing for determining FE[V.sub.1] and FVC was performed using a spirometer (AUTOSPIRO AS-600; Minato Medical Science Co Ltd; Osaka, Japan). The largest FE[V.sub.1] and FVC values from three maneuvers were analyzed. The residual volume was measured by the closed-circuit helium method, and the diffusing capacity of the lung for carbon monoxide (DLCO) was measured by the single-breath technique (CHESTAC-65V; Chest; Tokyo, Japan). The predicted values for the pulmonary function indexes were calculated based on the recommendations of the Japan Society of Chest Diseases. (12)
Study Design
During the initial screening, the subjects practiced progressive cycle ergometry on at least two occasions. When the subjects were seated on the cycle ergometer before each test, they were instructed on how to evaluate their breathlessness on the Borg scale (0 to 10) (13) written on the board in front of them. The instructions were briefly as follows: "This is a scale for rating your sensation of breathlessness. The No. 0 represents no breathlessness, and the No. 10 represents the strongest feeling of breathlessness you have ever experienced. Whenever you feel a change in your level of breathlessness during exercise, press a button you have and rate your perceived level of breathlessness at that time." In this way, the subjects were familiarized with the use of the Borg score during cycle exercise.
All eligible patients performed the exercise tests at the Kyoto University Hospital at around the same time in the morning on four separate days over a 2-week period. They were asked to stop the inhalation of their bronchodilator medications for at least 12 h before starting each exercise test. Patients who showed untoward clinical signs such as ECG changes during the tests were excluded from the present study.
On day 1, the symptom-limited progressive cycle ergometry was performed, and then the individual maximum work rate (Wmax) was determined as the highest work level reached. The exercise test was performed 60 min after inhaling 400 [micro]g (four puffs) salbutamol plus 80 [micro]g (four puffs) ipratropium bromide using a metered-dose inhaler with a spacer device (InspirEase; Schering-Plough KK; Osaka, Japan), (14) in order not to underestimate the Wmax.
The inhalation was performed using the following procedure. The spacer was attached to the metered-dose inhaler held in the mouth, and the canister was activated after the patient had exhaled to functional residual capacity. The patients inhaled slowly until total lung capacity was reached, and then the breath was held for at least 10 s. To ensure that the drugs were administered correctly, the inhalation technique was carefully observed.
On at least one occasion on other days between days 1 and 2, the subjects practiced the cycle endurance test at a constant work rate of 80% of the Wmax reached on the progressive cycle ergometry. The same constant work rate was used in the endurance tests performed on days 2, 3, and 4.
On days 2, 3, and 4, the cycle endurance tests were performed at 80% of the Wmax on the progressive cycle ergometry 40 min after inhaling 400 [micro]g salbutamol, 80 [micro]g ipratropium bromide, or an identical placebo in a randomized, double-blind, crossover fashion. The spirometric parameters then were assessed before inhalation, and at 30 and 60 min after inhalation. Prior to each spirometric measurement, the pulse rate and BP were measured using a sphygmomanometer (Yamasu 605P; Kenzmedico Co; Saitama, Japan) after at least 5 min of rest.
Exercise Tests
A calibrated, electrically braked cycle ergometer (Corival WLP-400; Lode; Groningen, the Netherlands) was used to conduct the symptom-limited progressive exercise test. The subjects breathed through a low-resistance unidirectional valve (Rudolph Face Mask Exercise Testing; Hans Rudolph Inc; Kansas City, MO) attached to their face mask. The patients began unloaded pedaling for 3 min, after which the workload was increased progressively by increments of 1 W every 3 s until the patient could no longer continue the required cadence of 40 cycles per minute due to severe dyspnea or exhaustion. The exercise data were recorded using an automated exercise testing system (Desktop Diagnostics/CPX; Medical Graphic Corporation; St. Paul, MN) that converts the breath-by-breath analog input into a digital form on-line. The minute ventilation (YE) and oxygen and carbon dioxide tension in the expired air were determined every eight breaths, and the mean VE, oxygen uptake (V[O.sub.2]) and carbon dioxide output (VC[O.sub.2]) then were calculated. The gas analyzer was calibrated just before the study with air and a standard reference gas mixture (ie, 15% oxygen and 5% carbon dioxide). The arterial oxygen saturation (Sa[O.sub.2]) was measured by pulse oximetry (N-200 pulse oximeter; Nellcor Inc; Hayward, CA), and the heart rate (HR) by electrocardiography (Life Scope 8; Nihon Koden Co; Tokyo, Japan). The analysis of the expired gas and the monitoring of the Sa[O.sub.2] and HR were continued for 3 min after the cessation of exercise. During exercise, symptoms of breathlessness were scored using the Borg scale. (13) In front of the cycle ergometer, a large board was set up with the Borg scale written on it. The patients rated their perceived level of breathlessness by pressing an electronic button whenever they felt a change in the level of their breathlessness. The Wmax was defined as the highest work level that was reached.
A submaximal endurance test was performed on the cycle ergometer at 80% of the Wmax reached on the progressive cycle ergometry. After unloaded pedaling for 3 min, the power output was increased to that work level. The patients continued cycling at the constant workload until the test was stopped according to the same criteria as the symptom-limited progressive cycle ergometry, and the endurance time was recorded. The highest values for V[O.sub.2], VC[O.sub.2], and VE attained during the submaximum test were determined using the mean values that were calculated every eight breaths. The Borg scores during exercise also were recorded on the endurance test as per the progressive cycle ergometry.
Statistical Analysis
The results are expressed as the mean [+ or -] SD. The dyspnea ratio was expressed as the ratio of the change in the Borg score to the endurance time. (9,15,16) Comparisons of the values between the placebo and the bronchodilators, and between salbutamol and ipratropium bromide were performed by a two-tailed, paired t test (parametric data) or a Wilcoxon signed rank test (nonparametric data). The relationships between the two sets of data were analyzed by the Pearson correlation coefficient test. A p value of < 0.05 was considered to be statistically significant.
RESULTS
The baseline characteristics of the 67 COPD patients are presented in Table 1. Their mean age was 70.4 [+ or -] 5.6 years, and the mean FE[V.sub.1] was 1.15 [+ or -] 0.41 L (44.2 [+ or -] 15.5% predicted). The effects of salbutamol and ipratropium bromide on pulmonary function and the results of the exercise performance tests are presented in Table 2. At 30 min, both bronchodilators produced significant improvements in FE[V.sub.1] and FVC compared to placebo (p < 0.001). When comparing the changes between the two bronchodilators, salbutamol caused significantly greater improvements than ipratropium bromide with respect to FE[V.sub.1] (p = 0.003) and FVC (p < 0.001).
With regard to exercise capacity, both bronchodilators significantly improved the endurance time by 29 s (15%; p < 0.001) and 27 s (14%; p < 0.001), respectively, in comparison to placebo. However, these values were not significantly different between salbutamol and ipratropium bromide (p = 0.71). This also has been shown to be true for the highest go2 and the highest VE values.
The highest dyspnea during exercise, as evaluated by the Borg score, was not significantly different between the placebo and bronchodilators. The dyspnea ratios derived from the change in the Borg score during exercise over the endurance time were significantly reduced by therapy with the bronchodilators (p < 0.001), although their effects were not significantly different (p = 0.23). During exercise, the highest HR and minimal Sa[O.sub.2] values also did not differ significantly between the bronchodilators.
The data concerning the relationships among the responses to the different bronchodilators are presented in Fig 1. Figure 1, top, A, shows the relationship of the difference in the endurance time between salbutamol and the placebo to the difference in the endurance time between ipratropium bromide and the placebo. These were moderately correlated with each other (r = 0.50; p < 0.001). However, a wide variability of individual values was found with a coefficient of determination ([r.sup.2]) value of 0.25. Figure 1, bottom, B, shows the relationship of the difference in FE[V.sub.1] at 30 min between salbutamol and the placebo with the difference between ipratropium bromide and the placebo. A significant, but moderate, relationship also was observed (r = 0.64; p < 0.001). A wide variability of individual values also was found with an [r.sup.2] value of 0.41.
[FIGURE 1 OMITTED]
Figure 2 shows the relationship between the change in airflow limitation and the change in the indexes on the exercise tests for each bronchodilator. The relationships of the difference in FE[V.sub.1] at 30 Din between bronchodilators and the placebo vs the differences in the endurance time (Fig 2, top, A), the highest V[O.sub.2]2 (Fig 2, middle, B), and the highest VE, (Fig 2, bottom, C) between bronchodilators and the placebo were nonexistent or weakly significant for both salbutamol and ipratropium bromide. Their correlation coefficients ranged from 0.14 to 0.37.
[FIGURE 2 OMITTED]
DISCUSSION
We found that salbutamol and ipratropium bromide had significant and similar effects on exercise capacity in stable patients with COPD, using the endurance time as an index of exercise capacity. In addition, the response to the different bronchodilators was shown to vary within individuals, for both FE[V.sub.1] and exercise capacity.
We demonstrated that the endurance time after therapy with salbutamol and ipratropium bromide were similar in 67 patients with COPD. Previous studies have addressed the conflicting results on whether these drugs could improve exercise capacity. In particular, the effects of [[beta].sub.2]-agonists on exercise capacity were mostly negative, (5,6) and furthermore, no dose-response relationship was demonstrated, (7) unlike the situation with the anticholinergic agents. (8)
One novelty of the present study was that we used submaximal endurance time as an index of exercise capacity, which was the most sensitive measure for detecting the small effects of oxitropium bromide in COPD patients. (9) O'Donnell et al (16) also demonstrated that the endurance time was reproducible and responsive to changes in COPD.
To the best of our knowledge, this is the first report demonstrating that both bronchodilators could improve laboratory exercise capacity similarly following a single administration in stable patients with COPD. Although their bronchodilating effects are considered to be similar in COPD patients, (2-4) the comparison of the effects of different types of bronchodilators on exercise capacity has been controversial. Leitch et al (17) reported that in 24 patients with chronic bronchitis the administration of 200 [micro]g salbutamol increased the 12-min walking distance significantly, but that 40 [micro]g ipratropium bromide did not. In contrast, Tobin et al (18) reported that in 12 patients with emphysema the administration of 40 [micro]g ipratropium bromide produced a greater maximal workload on progressive cycle ergometry, but that 400 [micro]g fenoterol did not. Based on a long-term view, Blosser et al (19) reported that 1 week of therapy with albuterol or ipratropium bromide had similar effects on the 12-min walking distance in 15 patients with COPD.
Liesker et al (20) recently analyzed the effects of bronchodilators on exercise capacity and pointed out six reasons for their negative results, as follows: (1) lack of clarity about whether the patients were actually limited by their ventilatory capacity; (2) the selection of the study population; (3) the dosing of the bronchodilator; (4) the potential negative effects of the bronchodilators; (5) the involvement of learning effects; and (6) the small number of patients included. We would also add to their list the type of exercise test that was performed. (9) We listed three studies that showed conflicting results about the effects of the different bronchodilators on exericse capacity, and which effects ,night have been partly caused by the small number of patients, the low dose administered, and the type of exercise test. Therefore, in the present study, we have changed these variables.
Dyspnea is an important outcome for patients with COPD. We used exertional dyspnea as a secondary outcome in the present study, because the question as to which bronchodilator would be more effective against dyspnea in COPD patients has not been resolved. We demonstrated that therapy with both salbutamol and ipratropium bromide reduced exertional dyspnea compared to the placebo, although their effects were similar. We used the dyspnea ratio of the change in the Borg score over the endurance time as an index, (9,15,16) because the highest Borg scores are usually the same after inhaling either the bronchodilators or the placebo. (16) The improvements in dyspnea during exercise induced by the bronchodilators in COPD are considered to be closely related to the reduction in dynamic hyperinflation. (16,21)
In the present study, a moderate relationship was observed between the response to the different bronchodilators for endurance time and airflow limitation (Fig 1). Previously, Hay et al (22) reported that the bronchodilating effects of different bronchodilators varied within individuals. The present study indicated that this was the same for endurance time. In addition, Fig 2 showed nonexistent or weak relationships between the effects of both bronchodilators on airflow limitation vs their effects on indexes for exercise tests. These results imply that different and complex mechanisms are responsible for the effects of the bronchodilators on exercise capacity vs airflow limitation in patients with COPD.
In patients with COPD, ventilatory limitation is considered to be the most important reason for stopping exercise. (20) It was anticipated that this limitation would be partially improved by the bronchodilators, although the bronchodilating mechanisms of both drugs are different. However, some patients may have stopped exercising due to nonventilatory reasons, such as leg muscle fatigue, cardiovascular limitation, motivation, or diminished DLCO. In these eases, the effects of the bronchodilators on exercise capacity may not be relevant. This may be one reason for the weak relationship between the effects of the bronchodilators on exercise capacity and those on airflow limitation. One limitation of the present study was that we should have evaluated the reasons for stopping exercise, or at least breathlessness or leg fatigue, separately. This might have added more information about the mechanism responsible for exercise limitation.
Some patients showed large reversibility in the present study. One reason may be that withdrawal of the bronchodilators for > 12 h before each test optimized their effects. Furthermore, as Anthonisen and Wright (23) reported, there are some COPD patients who have an exaggerated response to bronchodilators.
In conclusion, we demonstrated that therapy with both salbutamol and ipratropium bromide improved exercise capacity and reduced dyspnea similarly in patients with COPD. In addition, the effects of the different bronchodilators on exercise capacity varied within individuals, and a complex mechanism may be responsible for the different effects of the bronchodilators on exercise capacity vs airflow limitation. Therefore, the present study suggests that it might be difficult to decide whether inhaled [[beta].sub.2]-agonists or anticholinergic agents are better therapy for stable patients with COPD with regard to their effects on exercise capacity and dyspnea as treatment outcomes. Other outcome measures such as health-related quality of life, mortality, or utilization of health-care resources might show a difference between the two drug types.
REFERENCES
(1) Martin RJ, Kraft M. Combination therapy for asthma and chronic obstructive pulmonary disease. New York, NY: Marcel Dekker, 2000
(2) Ikeda A, Nishimura K, Koyama H, et al. Bronchodilating effects of combined therapy with clinical dosages of ipratropium bromide and salbutamol for stable COPD: comparison with ipratropium bromide alone. Chest 1995; 107:401-405
(3) Koyama H, Nishimura K, Ikeda A, et al. A comparison of the bronchodilating effects of oxitropium bromide and fenoterol in patients with chronic obstructive pulmonary disease. Chest 1993; 104:1743-1747
(4) Karpel JP. Bronchodilator responses to anticholinergic and beta-adrenergic agents in acute and stable COPD. Chest 1991; 99:871-876
(5) Iversen ET, Sorensen T, Heckscher T, et al. Effect of terbutaline on exercise capacity and pulmonary function in patients with chronic obstructive pulmonary disease. Lung 1999; 177:263-271
(6) Evald T, Keittelmann S, Sindrup JH, et al. The effect of inhaled terbutaline on FE[V.sub.1], FVC, dyspnoea and walking distance in patients with chronic obstructive lung disease. Respir Med 1992; 86:93-96
(7) Jaeschke R, Guyatt GH, Willan A, et al. Effect of increasing doses of beta agonists on spirometric parameters, exercise capacity, and quality of life in patients with chronic airflow limitation. Thorax 1994; 49:479-484
(8) Ikeda A, Nishimura K, Koyama H, et al. Dose response study of ipratropium bromide aerosol on maximum exercise performance in stable patients with chronic obstructive pulmonary disease. Thorax 1996; 51:48-53
(9) Oga T, Nishimura K, Tsukino M, et al. The effects of oxitropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease: a comparison of three different exercise tests. Am J Respir Crit Care Med 2000; 161:1897-1901
(10) Celli BR, Snider GL, Heffner J, et al. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152:S77-S120
(11) American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med 1994; 152:1107-1136
(12) Japan Society of Chest Diseases. The predicted values of pulmonary function testing in Japanese. Jpn J Thorac Dis 1993; 31:Appendix
(13) Borg GAV. Psychophysical basis of perceived exertion. Med Sci Sports Exerc 1982; 14:377-381
(14) Tobin MJ, Jenouri G, Danta I, et al. Response to bronchodilator drug administration by a new reservoir aerosol delivery system and a review of other auxiliary delivery systems. Am Rev Respir Dis 1982; 126:670-675
(15) Tsukino M, Nishimura K, Ikeda A, et al. Effects of theophylline and ipratropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. Thorax 1998; 53:269-273
(16) O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158:1557-1565
(17) Leitch AG, Hopkin JM, Ellis DA, et al. The effect of aerosol ipratropium bromide and salbutamol on exercise tolerance in chronic bronchitis. Thorax 1978; 33:711-713
(18) Tobin MJ, Hughes JA, Hutchison DC. Effects of ipratropium bromide and fenoterol aerosols on exercise tolerance. Eur J Respir Dis 1984; 65:441-446
(19) Blosser SA, Maxwell SL, Reeves-Hoche MK, et al. Is an anticholinergic agent superior to a 132-agonist in improving dyspnea and exercise limitation in COPD? Chest 1995; 108:730-735
(20) Liesker JJW, Wijkstra PJ, ten Hacken NHT, et al. A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 2002; 121:597-608
(21) Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153:967-975
(22) Hay JG, Stone P, Carter J, et al. Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. Eur Respir J 1992; 5:659-664
(23) Anthonisen NR, Wright EC. Bronchodilator response in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133:814-819
* From the Respiratory Division (Drs. Oga and Nishimura), Kyoto-Katsura Hospital, Kyoto, Japan; the Department of Respiratory Medicine (Drs. Tsukino, Sato, and Mishima), Graduate School of Medicine, Kyoto University, Kyoto, Japan; and the Department of Pulmonary Medicine (Dr. Hajiro), Kobe Nishi City Hospital, Kobe, Japan.
Manuscript received February 5, 2002; revision accepted December 11, 2002.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
Correspondence to: Toru Oga, MD, Respiratory Division, Kyoto-Katsura Hospital, 17 Yamadahirao, Nishikyo-ku, Kyoto 615-8256, Japan; e-mail: ogat@df7.so-net.ne.jp
COPYRIGHT 2003 American College of Chest Physicians
COPYRIGHT 2003 Gale Group