J P Mitchell(*); S-L Bates; K J Wiersema; M W Nagel; R W Morton mid J N Schmidt. Trudell Medical International, London, ON, Canada.
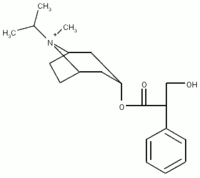
PURPOSE: To compare the performance of a new small volume VHC (AeroChamber[TM]-Plus - 149 ml- Monaghan Medical Corp, Plattsburgh, NY) with a larger VHC (OptiChamber[R] - 218 ml, Respironics, Cedar Grove, NJ) for the delivery of a combination [Beta]-agonist/anticholinergic formulation for the treatment of COPD (Combivent[R] 103 [micro]g/dose albuterol sulfate (SAL) + 18 [micro]g/dose ipratropium bromide (IPR) ex actuator mouthpiece (Boehringer-Ingelheim Pharmaceuticals Inc).
METHODS: Fine particle dose (FPD, particles [is less than] 4.7 [micro]m aerodynamic diameter) and total emitted dose (ED) were determined for each VHC (n = 5 devices for each group) by Andersen 8-stage impactor, operating at 28.3 [+ or -] 0.5 L/min. The quantities of SAL and IPR components collected in the impactor were determined by HPLC-UV spectrophotometry.
RESULTS: FPD and ED (% of label claim dose) were as follows. AeroChamber[TM]-Plus VHC: SAL - 56.2 [+ or -] 3.6%, 59.7 [+ or -] 4.1%; IPR - 47.0 [+ or -] 4.5%, 51.0 [+ or -] 5.0%, OptiChamber[R] VHC: SAL - 41.1 [+ or -] 3.3%, 43.5 [+ or -] 3.1%; IPR - 35.0 [+ or -] 6.0%, 38.0 [+ or -] 7.0%. The AeroChamber[TM]-Plus VHCs delivered significantly more of both components as either FPD or ED [unpaired t-test, p [is less than or equal to] 0.001].
CONCLUSION: Increased chamber volume does not necessarily correlate with improved FPD or ED with this formulation. Other considerations, such as internal geometry and inhalation valve design contribute to performance by controlling internal aerosol losses.
CLINICAL IMPLICATIONS: The differences observed with these specific devices may have significant clinical implications that require further study.
GRANT SUPPORT: Supported by Trudell Medical International
COPYRIGHT 2000 American College of Chest Physicians
COPYRIGHT 2001 Gale Group