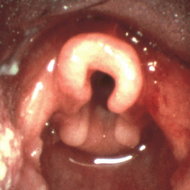
Laryngomalacia, or congenital laryngeal stridor, is the most frequent cause of noisy breathing in infants. The typical inspiratory, fluttery, sometimes hic-cup-like squeak of laryngomalacia results from turbulent airflow through the laryngeal airway. Upon inspiration, some or all of the supraglottic soft tissue structures, including rhe epiglottis, aryepiglottic folds and mucosa overlying the arytenoid cartilages, collapse inward and impede airflow. These flexible structures blow passively out of the laryngeal inlet upon exhalation, so there is no resistance to air egress and therefore no expiratory stridor. This most common congenital laryngeal anomaly accounts for 65-75% of cases of stridor in children under one year of age 1,2 and is eight times more common than tracheomalacia.
Despite its widespread prevalence, the etiology of laryngomalacia is unclear. Whether it represents an abnormality of laryngeal cartilage, mucosa or neuromuscular function remains under debate. One popular theory suggests a localized form of neuromuscular weakness, or laryngeal hypotonia, is responsible for the soft tissues' inability to resist the forces of airflow during respiration. Another theory implicates laryngeal mucosal inflammation secondary to gastroesophageal reflux, or, more specifically, laryngopharyngeal reflux. In his prospective study of infants with a new diagnosis of laryngomalacia, Giannoni et al found 64% to have pathologic gastroesophageal reflux.3 Matthews et al found pharyngeal acid exposure as judged by 24 hour dual-probe pH testing was found in 100% in children with laryngomalacia4 when only 66% had abnormal acid exposure in the esophagus. The link with reflux is also supported by histologie examination of mucosa from larynges of infants with laryngomalacia, which reveals a cellular infiltrate and submucosal edema consistent with reflux inflammation.5 It is theorized that contact with gastric contents may injure the supraglottic tissues sufficiently to allow inspiratory collapse. Conversely, laryngopharyngeal reflux may be the result, not the cause, of laryngomalacia, as the increased work of breathing generates an intraabdominal/intrathoracic pressure gradient sufficient to overcome the lower esophageal sphincter barrier. Whether it turns out that reflux is the primary or secondary event, several studies have documented it as a frequent comorbidity with laryngomalacia.6
The stridor associated with laryngomalacia fluctuates in intensity throughout the day and night, and most children have periods of quiet respiration. Stridor can occur at rest, with excitement, with sleep, and often with feeding. Although feeding times may be prolonged due to frequent pauses for breath, symptoms of aspiration are uncommon. The noisy breathing is often positional; supine positioning allows the epiglottic soft tissues to fall backward into the laryngeal inlet, leading to a common complaint of worsened obstruction during diaper changes. Interestingly, stridor usually lessens in intensity during crying.
The diagnosis of laryngomalacia is often made clinically. The onset of fluctuating, positional, inspiratory stridor in the first month of life is considered so typical for this common anomaly that the diagnosis is often assigned based upon the character of the stridor alone. A provisional diagnosis may be adequate in the uncomplicated patient who has unlabored breathing and is otherwise thriving. The definitive diagnosis is secured by direct visualization of the larynx during respiration. Flexible laryngoscopy can be accomplished in the otolaryngologist's office using a topical anesthetic. This technique not only confirms the diagnosis but also provides details regarding the type and degree of inspiratory collapse and the severity of associated reflux laryngitis. Other laryngeal disorders which cause inspiratory stridor, such as vocal cord paralysis, can be ruled out through this examination.
Laryngomalacia usually occurs as an isolated anomaly, but these infants are at risk for concomitant lesions elsewhere in the upper airway. Synchronous airway lesions, such as tracheomalacia, tracheal compression from anomalous thoracic vasculature, and congenital subglottic stenosis have been reported in up to 19% of children with laryngomalacia.7 Some of these lesions are clinically insignificant and are treated expectantly, but others may seriously impact upon respiratory status and require intervention. Suspicion for synchronous airway lesions is raised when the clinical picture varies at all from the norm for uncomplicated laryngomalacia. For example, since the stridor from laryngomalacia is purely inspiratory, biphasic stridor is worrisome and may be an indicator of pathology in the subglottic larynx or trachea. It is therefore very important that a thorough upper airway evaluation be performed when the degree of respiratory obstruction is moderate or severe, when the symptoms do not match the degree of laryngomalacia noted by flexible laryngoscopy in the office, or when symptoms atypical for laryngomalacia are noted.7
The expected clinical course for uncomplicated laryngomalacia entails the onset of stridor at approximately one to two weeks of age followed by slow, spontaneous resolution over the first year of life. Stridor at birth is unusual, raising concern of more severe congenital laryngotracheal anomalies, and should be evaluated before discharge from the newborn nursery. The degree of stridor typically increases as respiratory demand increases during early infancy and becomes most marked at six to eight weeks of age. After several months, the fluctuating stridor then gradually diminishes in frequency and intensity until complete resolution occurs. Most children are free of stridor by one year of age and almost all by eighteen months;7 the endoscopic appearance of the larynx is then normal. Persistence of symptoms later into childhood may represent ongoing reflux inflammation or an underlying neuromuscular disorder with laryngeal hypotonia (e.g., cerebral palsy).
The vast majority of infants will outgrow this disorder uneventfully. Complications arise when the degree of airway obstruction causes feeding difficulties or a significantly increased work of breathing. Poor feeding, failure to thrive, chronic retractions and tachypnea, apnea and cyanosis occur in 10% of cases8 and mandate intervention. Conservative management involves feeding and positioning strategies. Upright positioning in an infant seat during the daytime and lying the baby on his side in the crib is often helpful. Although airway obstruction from laryngomalacia is usually improved when the child is prone, the current American Academy of Pediatrics position on Sudden Infant Death Syndrome recommends against infants sleeping prone as a rule. Thickening formula when appropriate, and frequent, small feedings may also be of benefit.
Medical treatment of laryngomalacia is geared toward the elimination of laryngeal inflammation due to laryngopharyngeal reflux. The most commonly employed agents are H2 blockers (ranitidine, famotidine) and proton pump inhibitors (omeprazole, lansoprazole). Most children with laryngopharyngeal reflux do not demonstrate symptoms of excessive spitting up or emesis and do not require prokinetic agents such as metoclopramide. When antireflux therapy provides an improvement in upper airway status it is continued until symptoms of laryngomalacia begin to regress.
If complications of laryngomalacia persist despite medical therapy, direct laryngoscopy and bronchoscopy should be performed to confirm the diagnosis, assess for signs of uncontrolled reflux airway disease and rule out synchronous laryngotracheal lesions. If no other significant pathology is identified, surgical intervention is indicated. Before the late 1980s, failure to thrive, retractions, apnea and cyanosis from severe laryngomalacia was treated with tracheostomy. The airway obstruction was thereby bypassed and decannulation of the tracheostomy could be achieved once the disorder was outgrown. In rare instances, such as children with multiple comorbidities, in particular severe neurologic and cardiac disease, tracheostomy is still considered an appropriate intervention when more conservative measures fail.
Lane in 19849 and Seid10 in 1985 introduced a procedure by which portions of the obstructing laryngeal tissue are removed endoscopically, allowing an immediate improvement in the supraglottic airway. This technique, termed supraglottoplasty, employs the operating microscope to visualize the larynx, and microinstruments or the carbon dioxide laser to incise or excise the supraglottic soft tissue as needed. The actual procedure is tailored to the anatomy for each patient. If the aryepiglottic folds are foreshortened and tether the epiglottis posteriorly, they may simply be incised, allowing the epiglottis to move anteriorly away from the laryngeal lumen. If the mucosa overlying the arytenoids cartilages is redundant and collapses anteriorly into the glottic airway, one or two millimeters may be excised. Rarely do the cartilaginous structures require excision. The procedure is conservative; the intent is not to eliminate all stridor, but to improve the airway obstruction sufficiently as to allow frequent periods of quiet respiration, relieve retractions and apneic spells, and provide unimpeded breathing during feeding. Following a supraglottoplasty procedure, the airway is maintained by endotracheal intubation for a period ranging from a few hours to overnight. In some children, the airway improvement is so marked as to allow extubation in the operating room. Patients are then monitored for airway obstructive symptoms during feeding, crying and sleep. In the absence of comorbid disorders, children are routinely discharged home on the first post-operative day.
The success of supraglottoplasty, as defined by a resolution of feeding difficulties, apnea, and respiratory distress, is excellent in infants with laryngomalacia alone. Large series report success rates of 89%11 to 100%.12 Children with underlying neurologic or neuromuscular disorders are less likely to have a complete resolution of upper airway obstruction. " Denoyelle et al noted their success rate of 89% dropped to 50% in their patients with associated congenital abnormalities.11
Complications reported with the supraglottoplasty procedure include postoperative laryngeal edema, aspiration and supraglottic stenosis and are reported to occur in 0%12,14 to 8%15 of patients. In an effort to limit potential complications of the procedure, two institutions each performed a series of unilateral supraglottoplasty procedures and found their success rates unchanged at 93% and 94%, respectively."14,15 This supports the sentiment that "less is more," and is one reason why the procedure is intentially conservative. Another is that revision surgery with further excisions can be performed, although this is rarely necessary in our experience. Most importantly, stridor related to residual laryngomalacia will improve spontaneously over rime, because that is the natural progression of the disease, as long as the complicating factors of respiratory distress and failure to thrive have been relieved.
In general, laryngomalacia is a common and self-limited disorder. Most infants will be squeaky but otherwise well, with periods of quiet, restful respiration, gaining weight, and thriving, and not requiring intervention. Many others will have more significant symptoms but a stable upper airway status if associated laryngopharyngeal reflux is addressed. Surgical intervention is reserved for the few with manifestations of moderate to severe upper airway obstruction. In all infants, it is important to recognize when signs or symptoms stray from those expected for uncomplicated laryngomalacia.
REFERENCES
1. Holinger, LD. Etiology of stridor in the neonate, infant, and child. Ann Otol Rhinol Laryngol 1980; 89:397-400.
2. Cotton RT, Reilly JS. Congenital Malformations of the larynx. In: Bluestone CD, Stool SE, eds. Pediatrie Otolaryngology. Vol 2. Philadelphia: WB Saunders, 1983:1300-1.
3. Giannoni C, Sulek M, Friedman EM, et al. Gastroesophageal reflux association with laryngomalacia: A prospective study. Int J Pediatr Otorhinolaryngol 1998;43:11-20.
4. Matthews BL, Little JP, McGuirt WF Jr, et al. Reflux in infants with laryngomalacia: results of 24-hour double-probe pH monitoring. Otolaryngol Head Neck Surg 1999;120:860-4.
5. Chandra RK, Gerber ME, Holinger LD. Histological insight into the pathogenesis of severe laryngomalacia. Int J Pediatr Qtorhinolaryngol 2001;61:31-8.
6. Bibi H, Khvolis E, Shoseyov D, et al. The prevalence of gastroesophageal reflux in children with tracheomalacia and laryngomalacia. Chest 2001;119:409-13.
7. Olney DR, Greinwald JH Jr, Smith RJH, et al. Laryngomalacia and its treatment. Laryngoscope 1999;109:1770-5.
8. Kavanagh KT, Babin RW. Endoscopic surgical management for laryngomalacia: Case report and review of the literature. Ann Otol Rhinol laryngol 1987;96:650-3.
9. Lane RW, Weider DL, Steinern C, et al. Laryngomalacia: A review and case report of surgical treatment with resolution of pectus excavatum. Arch Otolaryngol 1984; 110:546-51.
10. Seid AB, Park SM, Kearns MJ, et al. Laser division of the aryepiglottic folds for severe laryngomalacia. Int J Pediatr Otorhinolaryngol 1985; 10:153-8.
11. Denoyelle F, Mondain M, Gresillon N, et al. Failures and complications of supraglottoplasty in children. Arch Otolaryngol Head Neck Surg 2003;129:1077-80.
12. Senders CW, Navarrete EG. Laser supraglottoplasty for laryngomalacia: Are specific anatomical defects more influential than associated anomalies on outcome? Int J Pediatr Otorhinolaryngol 2001;57:235-44.
13. Toynton SC, Saunders MW, Bailey CM. Aryepiglottoplasty for laryngomalacia: 100 consecutive cases. J Laryngol Otol 2001;115:35-8.
14. Kelly SM, Gray SD. Unilateral endoscopic supraglottoplasty for severe laryngomalacia. Arch Otolaryngol Head Neck Surg 1995;121:1351-4.
15. Reddy DK, Mart BH. Unilateral vs bilateral supraglottoplasty for severe laryngomalacia in children. Arch Otolaryngol Head Neck Surg 2001;127:694-9.
SHARON E. GIBSON, MD
Sharon E. Gibson, MD, is Assistant Clinical Professor, Department of Surgery, Brown Medical School; Assistant Clinical Professor, Department of OtolaryngologyHead and Neck Surgery, Tufts University School of Medicine, and Director of Pediatric Otolaryngology, Hasbro Children's Hospital.
CORRESPONDENCE:
Sharon E. Gibson, MD
University Otolaryngology
130 Waterman St.
Providence, RI 02906
Phone:(401)274-3277
Fax:(401)274-0672
e-mail: sgibson@univoto.net
Copyright Rhode Island Medical Society Oct 2004
Provided by ProQuest Information and Learning Company. All rights Reserved