Leishmaniasis is endemic in 88 countries throughout Africa, Asia, Europe, and North and South America. (1) There are an estimated 12 million cases worldwide, with 1.5 to 2 million new cases each year. Although the incidence of leishmaniasis is greater in the Old World than in the New World, the U.S. traveler is most likely to contract this disease in Latin America. Fifty to 100 cases of New World cutaneous leishmaniasis are diagnosed each year in the United States. They are contracted mainly in Peru and Brazil, although the disease is endemic and can be contracted in any country from Mexico to Argentina, except Uruguay and Chile. (2) There also is an endemic focus in Texas. Leishmaniasis is a disease associated with rural areas and poverty, but it has adapted to the urban environment as well.
In World War II, there was a high incidence of leishmaniasis and sandfly fever in troops deployed to the Persian Gulf region. In the Gulf War (1990 to 1991), approximately 697,000 U.S. troops were deployed in this region. Only 19 cases of cutaneous leishmaniasis and 12 cases of visceral disease were diagnosed in this group. The improvement came about because of the use of insecticides and repellents, lower transmission rates in the summer, and more time spent in urban areas. (3,4) About 150 cases of leishmaniasis have reportedly been diagnosed in U.S. soldiers serving in Iraq in 2003, and more are expected. (5) Preliminary data on 22 cases of cutaneous leishmaniasis contracted by American troops in Afghanistan, Kuwait, and Iraq and treated at Walter Reed Army Medical Center between August 2002 and September 2003 were recently released. (6) The majority of these persons were infected with Leishmania major in urban areas of Iraq after a median period of deployment of 60 days.
The Leishmania protozoan was first described in 1903 by Leishman and Donovan, working separately. (2) Since then, this organism has been found to be a complex grouping of species, at least 20 of which cause infections in humans. Some species cause visceral leishmaniasis, some cause cutaneous disease, and some cause both. Visceral leishmaniasis is a systemic infection characterized by fever, weight loss, and hepatosplenomegaly, and it is usually fatal without treatment. This article focuses on cutaneous leishmaniasis, the more common form of the disease.
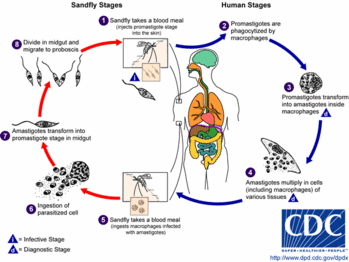
Life Cycle and Vector
The promastigote form of the parasite is a motile form with an anterior flagellum that develops in the sandfly, the insect vector. The promastigote form develops into a metacyclic infectious form over approximately 10 days. The parasite enters the human host with the bite of the sandfly and is pulled into macrophages by ingestion. Leishmania are able to survive the acidic environment of the lysosome and become amastigote forms. These forms are obligate, intracellular, non-motile, and about 2.5 to 7 microns in diameter. It is this amastigote form that causes disease in humans and affects cellular immunity. Eventually, a sandfly will pick up this form while feeding, and it will develop into the promastigote form again in the insect.
The sandfly vector is a 2-mm long, hairy fly of the genus Phlebotomus in the Old World and Lutzomyia in the New World. These flies are able to pass through the usual netting used for mosquitoes. Sandflies are found around human habitations and breed in specific organic wastes such as feces, manure, rodent burrows, and leaf litter. (7)
Cutaneous Leishmaniasis
Cutaneous leishmaniasis occurs in the New World and the Old World. Old World disease primarily is caused by Leishmania tropica in urban areas and Leishmania major in dry desert areas. The two subgenera of interest in Latin America are Leishmania leishmania (e.g., Leishmania mexicana, Leishmania amazonensis, Leishmania chagasi) and Leishmania viannia (e.g., Leishmania panamensis, Leishmania braziliensis, Leishmania guyanensis). The incubation period is two to eight weeks, although longer periods have been noted. The disease begins as an erythematous papule at the site of the sandfly bite on exposed parts of the body. The papule increases in size and becomes a nodule. It eventually ulcerates and crusts over. The border is usually raised and distinct. There may be multiple lesions, especially when the patient has encountered a nest of sandflies. The ulcer is typically large but painless unless there is secondary bacterial or fungal infection.
Old World leishmaniasis and L. mexicana lesions tend to heal spontaneously in months, but L. braziliensis may take years to heal. After healing, a depressed scar remains that is usually round but can be irregular. Figure 1 shows a typical leishmaniasis lesion before treatment. Satellite lesions with a nodular lymphangitis resembling sporotrichosis have been described.
Cutaneous leishmaniasis can become disseminated (diffuse cutaneous leishmaniasis), especially in immunosuppressed persons. This illness can go on for years and does not heal spontaneously. Patients with human immunodeficiency virus (HIV) infection are particularly susceptible. Other unusual types of cutaneous disease include leishmaniasis recidivans, in which small nodules develop around a healed scar, and post-kala-azar dermal leishmaniasis, in which widespread cutaneous lesions arise after a visceral infection. These conditions occur primarily in the Old World.
The mucosal form usually occurs after an initial cutaneous infection. Ninety percent of cases of mucosal leishmaniasis are found in Brazil, Bolivia, and Peru, and they usually begin in the nose or palate. (8) Lesions progress to destruction of mucosa and even cartilage. They result in scarring and disfigurement and can cause pulmonary aspiration and death. Table 1 lists the differential diagnosis for cutaneous and mucosal leishmaniasis. (9)
Diagnosis
When physicians assess a patient with suspected leishmaniasis in the United States, the travel and military histories are most important. Patients who served in the military in the Middle East can return with this infection. Risk factors for HIV should be solicited, including sexual encounters, intravenous drug use, and blood transfusions obtained abroad.
The basic diagnostic tests are summarized in Table 2. Cutaneous scraping is the simplest and most common test, but it is only 70 to 75 percent sensitive. (2) Proper cleaning and drying of the site are essential before scraping. Scrapings are made from the center and the margin of the ulcer. L. mexicana yields more organisms than L. braziliensis, and older lesions (more than four months) have fewer parasites than newer ones.
Multiple slides should be made. They are fixed with methanol, stained with Giemsa, and examined under oil immersion. Amastigotes are seen in monocytes or extracellularly. Slides must be examined completely before they can be called negative. It is important to see the nucleus and the rod-shaped kinetoplast, a mitochondrial structure containing extranuclear DNA, to diagnose leishmaniasis. The kinetoplast differentiates Leishmania from other small organisms such as Histoplasma.
Treatment
Treatment with antimonials will heal lesions faster and prevent relapse, local dissemination, mucosal disease (usually), and transmission. Not all lesions require treatment. Old World disease tends to be self-healing, and systemic treatment seldom is used. New World lesions more often require systemic treatment. Table 3 gives reasons to consider early treatment.
Pentavalent antimony remains the treatment of choice. It is thought to work by inhibition of adenosine triphosphate synthesis. The antimonial agent used in the United States is sodium stibogluconate (Pentostam). This drug is available from the Centers for Disease Control and Prevention (CDC), which also can assist with the investigational new drug paperwork.
Pentavalent antimonials have a high incidence of side effects, but the latter are reversible. In a military study,10 96 subjects with leishmaniasis (83 cases were cutaneous) were treated for 20 to 28 days and followed for one year. Side effects included aching, arthralgia, fatigue, gastrointestinal upset, elevation of amylase, lipase, and liver enzyme levels, leukopenia, anemia, and electrocardiographic abnormalities.
In general, more adverse reactions are expected in patients with liver and renal impairment, cardiac arrhythmias, and prolonged QT intervals; in small children; in pregnant and breastfeeding women; and in obese, elderly, and immunocompromised patients. There are no known drug interactions, but hepatotoxic drugs, including alcohol, and drugs that prolong the QT interval should be avoided. (10) Because pentavalent antimony is difficult to use, consultation with an infectious disease specialist and the CDC is recommended before using it.
After 20 days of treatment with pentavalent antimonials, there is usually evidence of healing, but lesions may not be re-epithelialized completely. They generally go on to heal. Healing is determined by a healed appearance at two months, no relapse at 12 months, and no subsequent mucosal disease. Mucosal disease requires a longer treatment course and is more difficult to cure.
Trauma can reactivate the disease, so elective surgery is contraindicated for one year, and pregnancy is inadvisable for one to two months after treatment. The aching, which can be debilitating, usually resolves one week after treatment is finished but can take up to two months to go away.
Table 4 includes the recommended dosage of pentavalent antimony and other treatment options. (11-19) A recent randomized, double-blind, placebo-controlled study of more than 200 patients with cutaneous L. major demonstrated that oral fluconazole (Diflucan) in a dosage of 200 mg daily for six weeks was a well-tolerated and effective treatment option. (18) [SOR B, single randomized controlled trial (RCT)].
The CDC is ready to assist with the diagnosis and treatment of leishmaniasis and can be contacted at 404-488-4050 or 404-639-3670.
Prevention
Vaccine development is under way. The combination of killed promastigotes plus bacille Calmette-Guerin vaccine is being tested in Iran, Sudan, and Ecuador. (20) Avoiding sandflies is important but difficult, because they have adapted to urban environments. The use of insecticides in endemic areas is important for travelers. House and space spraying have reduced sandfly populations, and fine-weave pyrethroid-impregnated bed-nets have been used in Burkina Faso, Sudan, and Columbia. Destruction of rodent reservoirs by pumping insecticides into rodent burrows has had limited success. (7)
A recent randomized study in Venezuela evaluated the effectiveness of pyrethroid-impregnated curtains in an urban area with an incidence of cutaneous leishmaniasis of 4 percent. In 569 homes, 2,913 inhabitants were included in this study. Use of the curtains reduced the sandfly population and, 12 months after the installation of these curtains, the incidence of cutaneous leishmaniasis dropped to zero. (21) [SOR B, single RCT]
Figure 1 provided by Eric J. Sheltra.
The authors indicate that they do not have any conflicts of interest. Sources of funding: none reported.
REFERENCES
(1.) Dedet JP, Pratlong F. Leishmaniasis. In: Manson P, Cook GC, Zumla A, eds. Manson's Tropical diseases. 21st ed. London: Saunders, 2003:1339-64.
(2.) Herwaldt BL. Leishmaniasis. Lancet 1999;354: 1191-9.
(3.) Cope SE, Schultz GW, Richards AL, Savage HM, Smith GC, Mitchell CJ, et al. Assessment of arthropod vectors of infectious diseases in areas of U.S. troop deployment in the Persian Gulf. Am J Trop Med Hyg 1996;54:49-53.
(4.) Hyams KC, Hanson K, Wignall FS, Escamilla J, Oldfield EC 3d. The impact of infectious diseases on the health of U.S. troops deployed to the Persian Gulf during operations Desert Shield and Desert Storm. Clin Infect Dis 1995;20:1497-504.
(5.) McNeil DG Jr. A region inflamed: medicine; hundreds of U.S. troops infected by parasite borne by sand flies, Army says. New York Times December 6, 2003:A,8.
(6.) Centers for Disease Control and Prevention (CDC). Cutaneous leishmaniasis in U.S. military personnel--Southwest/ Central Asia, 2002-2003. MMWR Morb Mortal Wkly Rep 2003;52:1009-12.
(7.) Lane RP. Phlebotomine sandflies. In: Manson P, Cook GC, Zumla A, eds. Manson's Tropical diseases. 21st ed. London: Saunders, 2003:1733-41.
(8.) WHO fact sheet. The leishmaniases and leishmania/ HIV coinfections. Accessed October 16, 2003, at: http://www.who.int/inf-fs/en/fact116.html.
(9.) Magill AJ. Leishmaniasis. In: Hunter GW, Strickland GT, eds. Hunter's Tropical medicine and emerging infectious diseases. 8th ed. Philadelphia: Saunders, 2000:665-87.
(10.) Aronson NE, Wortmann GW, Johnson SC, Jackson JE, Gasser RA Jr, Magill AJ, et al. Safety and efficacy of intravenous sodium stibogluconate in the treatment of leishmaniasis: recent U.S. military experience. Clin Infect Dis 1998;27:1457-64.
(11.) Martinez S, Marr JJ. Allopurinol in the treatment of American cutaneous leishmaniasis. N Engl J Med 1992;326:741-4.
(12.) Velez I, Agudelo S, Hendrickx E, Puerta J, Grogl M, Modabber F, et al. Inefficacy of allopurinol as monotherapy for Colombian cutaneous leishmaniasis. A randomized, controlled trial. Ann Intern Med 1997;126:232-6.
(13.) Esfandiarpour I, Alavi A. Evaluating the efficacy of allopurinol and meglumine antimoniate (Glucantime) in the treatment of cutaneous leishmaniasis. Int J Dermatol 2002;41:521-4.
(14.) Llanos-Cuentas A, Echevarria J, Cruz M, La Rosa A, Campos P, Campos M, et al. Efficacy of sodium stibogluconate alone and in combination with allopurinol for treatment of mucocutaneous leishmaniasis. Clin Infect Dis 1997;25:677-84.
(15.) Navin TR, Arana BA, Arana FE, de Merida AM, Castillo AL, Pozuelos JL. Placebo-controlled clinical trial of meglumine antimonate (glucantime) vs. localized controlled heat in the treatment of cutaneous leishmaniasis in Guatemala. Am J Trop Med Hyg 1990;42:43-50.
(16.) Velasco-Castrejon O, Walton BC, Rivas-Sanchez B, Garcia MF, Lazaro GJ, et al. Treatment of cutaneous leishmaniasis with localized current field (radio frequency) in Tabasco, Mexico. Am J Trop Med Hyg 1997;57:309-12.
(17.) Gurei MS, Tatli N, Ozbilge H, Erel O, Seyrek A, Kocyigit A, et al. Efficacy of cryotherapy and intra-lesional pentostam in treatment of cutaneous leishmaniasis. J Egypt Soc Parasitol 2000;30:169-76.
(18.) Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med 2002;346:891-5.
(19.) Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. BMJ 2003; 326:377-82.
(20.) Sharifi I, FeKri AR, Aflatonian MR, Khamesipour A, Nadim A, Mousavi MR, et al. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet 1998;351:1540-3.
(21.) Kroeger A, Avila EV, Morison L. Insecticide impregnated curtains to control domestic transmission of cutaneous leishmaniasis in Venezuela: cluster randomised trial. BMJ 2002;325:810-3.
WILLIAM H. MARKLE, M.D., and KHALDOUN MAKHOUL, M.D. University of Pittsburgh Medical Center, McKeesport Hospital, McKeesport, Pennsylvania
WILLIAM H. MARKLE, M.D., is assistant professor of family medicine at the University of Pittsburgh School of Medicine and family practice residency program director at the University of Pittsburgh Medical Center (UPMC), McKeesport, Pa. He is a graduate of the Penn State Milton S. Hershey Medical Center, Hershey, Pa., and completed a residency at Virginia Commonwealth University, Blackstone Family Practice Center, Blackstone, Va. He received a diploma in tropical medicine and hygiene at Mahidol University, Bangkok, Thailand.
KHALDOUN MAKHOUL, M.D., is currently in private practice in Easton, Pa. He is a graduate of the University of Aleppo Faculty of Medicine, Aleppo, Syria, and completed a family practice residency at UPMC McKeesport.
Address correspondence to William H. Markle, M.D., University of Pittsburgh Medical Center, McKeesport Family Practice Residency, 3518 Fifth Ave., Pittsburgh, PA 15261 (e-mail: marklew@upmc.edu). Reprints are not available from the authors.
COPYRIGHT 2004 American Academy of Family Physicians
COPYRIGHT 2004 Gale Group