QUESTION: Recently, the surgical site infection (SSI) rate among our OR patients has been increasing. We would Like to have more information on what pathogens cause SSIs. Are they from the patient or from the surgical suite? What are the risk factors related to SSIs?
ANSWER: According to the National Nosocomial Infections Surveillance System of the Centers for Disease Control and Prevention (CDC), pathogens that have been identified in SSIs include
* Bacteroides fragilis;
* Candida albicans;
* coagulase-negative staphylococci;
* enterobacter species;
* enterococcus species;
* Escherichia coli;
* group D streptococci (ie, nonenterococci), other streptococcus species, and other gram-positive aerobes;
* Klebsiella pneumoniae;
* Proteus mirabilis;
* Pseudomonas aeruginosa; and
* Staphylococcus aureus. (1)
Many of the pathogens causing an SSI are internal sources (ie, endogenous flora) that may come from the patient's own skin, mucous membranes, or hollow viscera. External sources (ie, exogenous) also can cause SSIs. These include surgical team members or equipment, instruments, or supplies on the sterile field used during the surgical procedure. (2)
Patients at the greatest risk for developing an SSI are those who
* are immunocompromised;
* are malnourished;
* are obese;
* are older;
* are undergoing a prolonged hospital stay;
* have blood transfusions with certain blood products;
* have diabetes and experience increased glucose levels (ie, greater than 200 mg/dL) in the immediate postoperative period; or
* smoke.
Preoperative colonization of the nares with Staphylococcus aureus also can increase the risk of contracting an SSI. (1)
Knowing which patients are most at risk for developing SSIs and understanding the possible sources of pathogen transmission may help surgical team members protect patients and control and prevent SSIs. Using antibiotic prophylaxis when appropriate, maintaining patients' temperatures, controlling patients' glucose levels, using clippers to remove hair, and adhering to the principles of asepsis are proactive measures for preventing SSIs.
QUESTION: We have pregnant health care workers (HCWs) who occasionally care for patients with infectious diseases. Is it safe for pregnant staff members to care for patients with infectious diseases? What diseases should pregnant HCWs be concerned about? What precautions should staff members take?
ANSWER: It is important to ensure the safety of all staff members, including those who are pregnant, when they care for patients with infectious diseases. The CDC's "Guideline for infection control of healthcare personnel, 1998" and the Association for Professionals in Infection Control and Epidemiology Text of Infection Control and Epidemiology discuss three principles of caring for patients with infectious diseases.
* When caring for patients with infectious diseases, pregnant HCWs are at no greater risk of acquiring an infection than HCWs who are not pregnant.
* Risk reduction for HCWs includes obtaining vaccinations against vaccine preventable diseases before pregnancy.
* All HCWs should adhere to standard and transmission-based precautions (eg, isolation) no matter what their immune status? (3,4)
One group of researchers determined that, on average, a woman's first prenatal visit occurs when she is between 7.5 and 10.9 weeks of gestation. (5) Another researcher identified that fetal organ development begins as early as three weeks gestation. (6) Exposure to an infectious disease, therefore, already may have taken place by the time a woman finds out she is pregnant. (7) This information reinforces the importance of preventative protection at all times.
Some examples of communicable diseases that may pose a particular concern for pregnant HCWs' unborn children are parvovirus B19, rubella, and cytomegalovirus (CMV).
* Parvovirus B19 (ie, erythema infectiosum, fifth disease) is a disease that usually affects children but also can affect adults, it occurs most often in late winter or early spring and is highly contagious but will spontaneously resolve in seven to 10 days. (8) One of the characteristics is erythema of the cheeks (ie, slapped-face look). The period of communicability is greatest before onset of the rash; however, patients who have aplastic crisis are communicable for one week after symptoms appear. Patients who are immunosuppressed and have chronic infection and severe anemia may be communicable for months to years. In approximately 10% of intrauterine infections, parvovirus may cause fetal anemia, which may result in hydrops fetalis and fetal death. Parvovirus is transmitted by contact with respiratory secretions. (9) Standard precautions should be followed for this disease. (10) Currently, there is no available vaccine to prevent parvovirus.
* Rubella (ie, German measles) can develop in children from five to nine years of age, adolescents, and young adults and usually occurs in winter and spring. The rash that presents may resemble that of measles or scarlet fever; however, 50% of patients may not develop a rash at all. (8) The period of communicability is approximately one week before and four days after the rash appears, and the disease is highly communicable. Rubella may cause fetal anomalies. Congenital rubella syndrome occurs in about 90% of infants born after acquired, confirmed rubella occurs during the first trimester. Transmission occurs via contact with nasopharyngeal secretions. (9) Isolation is required and droplet precautions must be followed. (10) A live virus vaccine is used for immunization. (8)
* Cytomegalovirus occurs in approximately 40% of all adults. (8) By age 70 to 80, 80% to 100% of adults have been infected with CMV. Patients may be asymptomatic or may have symptoms of fever, malaise, myalgia, and hepatitis. The period of communicability may be months to several years after primary infection. Transmission is by exposure to body fluids, such as saliva, blood, breast milk, and vaginal or seminal secretions. (11) As a result of intrauterine infection, CMV may cause the fetus to develop hearing loss or congenital syndrome. (9) Standard precautions should be followed for this disease. (10) Currently, there is no available vaccine.
Pregnant HCWs should avoid caring for patients with parvovirus B19, rubella, and CMV. All staff members should follow standard and droplet precautions when caring for patients with these infectious diseases.
Standard precautions are applied to all patients being cared for in health care facilities, regardless of diagnoses. This applies to working with all patient body fluids (eg, blood, secretions, excretions) except sweat, nonintact skin, and mucous membranes. Gown, gloves, and mask or eye protection should be worn as needed. Hand washing should be strictly enforced between patient or body fluid contact, after removing gloves, and any other time it is needed. Hand washing should be performed promptly and thoroughly.
Droplet precautions require the use of a surgical mask when working within 3 ft of patients who have a disease categorized as a droplet infection. (11)
Health care facilities should provide programs to assist all employees in reducing their risk for acquiring infections. Infection control programs should include providing vaccinations, proper personal protective equipment, and education.
QUESTION: We use multiple-use vials throughout our perioperative area. What is considered a multiple-use vial? Can they become contaminated and cause an infection? How should they be prepared for use? How should they be stored and maintained? Should they be dated when opened? What types of multiple-use medications are more Likely to cause an outbreak of infections?
ANSWER: An accurate description of a multiple-use vial is a vial that has an antibacterial preservative and can be used more than once according to the manufacturer's recommendations (eg, insulin, heparin). The label on the vial must state that the vial is for multiple use.
Serious outbreaks of infection can occur as a result of using contaminated vials. Recently, two deaths were reported that were caused by the injection of contrast media contaminated with Pseudomonas aeruginosa. The vial in this report had been used for 41 patients during an eight-day period. (12) The Joint Commission for the Accreditation of Healthcare Organizations requires that facilities actively use steps to prevent or reduce the transmission of nosocomial infections in patients. (13)
When preparing a multiple-use vial for use, a HCW should wash his or her hands before beginning the task. The stopper should be cleaned with 70% alcohol before any device (eg, needle, spike) is inserted into the vial. A sterile device should be used to access the multiple-use vial. Avoid touching the stopper during the process. The vial should be discarded if sterility is compromised. When the vial is opened, adhere to the manufacturer's expiration date if listed. The vial must contain a preservative and show no evidence of contamination for it to be used as a multiple-use vial. Do not use a vial if it
* contains particulate matter, precipitates, turbidity, or discoloration;
* is mislabeled;
* appears to be unusable; or
* is suspected to be contaminated.
Discard any vial suspected of being contaminated. (14) Vials should be refrigerated after they are opened. There is no requirement for dating a multiple-use vial after it is opened. Vials should be stored in a safe area where tampering or possible damage cannot occur.
Multiple-use medications containing lipids and those that contain preservative-free solutions have been reported to be a more likely cause of infections. Filters with spikes also may become contaminated. Using spikes may give HCWs a false sense of security, resulting in inappropriate handling of vials. (15)
Although multiple-use vials may be cost-effective, they may pose a risk for transmitting infection. Good aseptic technique must be used when vials are being prepared and used by more than one HCW. Safe storage of multiple-use vials also is important.
QUESTION: We recently had a patient with an ulcer on his forearm that needed to be debrided. The pathology report came back with a diagnosis of leishmaniasis. None of our staff members have heard of this disease. What is it? Where does it come from? Could we contract leishmaniasis by caring for a patient with the disease? How is it treated?
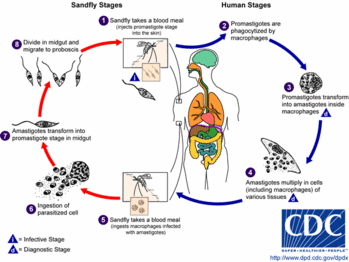
ANSWER: Cutaneous leishmaniasis is a vector-borne parasitic disease. It is transmitted by a bite from the female phlebotomine sand fly. (16) If symptoms develop from the bite, they may appear weeks to months after exposure. Cutaneous leishmaniasis skin lesions, which can be chronic and disfiguring, may progress from papules to nodules to ulcerative lesions but may remain as nodules or plaques. Lesions that become ulcerative need to be debrided.
The phlebotomine sand fly is endemic in many parts of the world, including Afghanistan, Kuwait, and Iraq. People who travel through these areas are at risk for sand fly bites. Military and civilian personnel in these areas also may be at risk for bites. Leishmaniasis does not appear to be a disease that is transmissible from person to person. Standard precautions should be used in caring for patients with this disease. Using these precautions protects caregivers and patients.
Treatment for cutaneous leishmaniasis is pentavalent antimonyal compound sodium stibogluconate (ie, pentostam). This medication is not licensed for general use in the United States. Military personnel who are in endemic areas and develop symptoms are evacuated to Waiter Reed Army Medical Center (WRAMC), Washington, DC, for treatment. Pentostam is provided by WRAMC under investigational new drug (IND) protocols that are governed by the surgeon general of the Army in conjunction with the US Food and Drug Administration (FDA). Civilian personnel who are working or have worked in these endemic areas and develop these symptoms are treated by their own health care provider who should contact the CDC's medication service at (404) 639-3670. The CDC has an IND protocol separate from the FDA for treatment of civilians with cutaneous leishmaniasis. There is no FDA-approved vaccine or prophylactic medication for the prevention of cutaneous leishmaniasis. (17)
For answers to your questions contact the Center for Nursing Practice at (800) 755-2676 x 334 or send an e-mail to consult@aorn .org. AORN's 2004 Standards, Recommended Practices, and Guidelines are now available.
NOTES
(1.) A J Mangram et al, "Guidelines for prevention of surgical site infection, 1999," American Journal of Infection Control 27 (April 1999) 97-132.
(2.) N Tomaselli, "Prevention and treatment of surgical-site infections," Infection Control Resource, http://www.infectioncontrol resource.org/IC_Issue5/V1.html (accessed 20 April 2004).
(3.) E A Bolyard et al, "Guideline for infection control in healthcare personnel, 1998," Infection Control and Hospital Epidemiology 19 (June 1998) 407-463.
(4.) "The pregnant healthcare worker," in APIC Text of Infection Control and Epidemiology, third ed (Washington, DC: Association for Professionals in Infection Control and Epidemiology, 2002) 1-9.
(5.) T A Von Busch et al, "Feasibility of maternity protection in early pregnancy, Journal of Occupational and Environmental Health 8 (October-December 2002) 328-331.
(6.) L M Frazier, T L Jones, "Managing patients with concerns about workplace reproductive hazards," Journal of American Medical Women's Association 55 (Spring 2000) 80-83, 105.
(7.) J M Lanson, "The pregnant healthcare worker," in Infection Connection Across the Health Care Continuum (Washington, DC: Association for Professionals in Infection Control and Epidemiology, 2004) 5.
(8.) K M Folk et al, Handbook of Infectious Diseases (Springhouse, Pa: Springhouse, 2000) 100.
(9.) A S Benenson, ed, Control of Communicable Diseases Manual, 1995, 16th ed (Washington, DC: American Public Health Association, 1995) 172-174.
(10.) J Jennings, J Wideman, eds, APIC Text of Infection Control and Epidemiology, third ed (Washington, DC: Association for Professionals in Infection Control and Epidemiology, 2002) 262-275.
(11.) "Herpes viruses" in APIC Text of Infection Control and Epidemiology, third ed (Washington, DC: Association for Professionals in Infection Control and Epidemiology, 2002) 1-4.
(12.) F Mattner, P Gastmeier, "Bacterial contamination of multiple-dose vials: A prevalence study," American Journal of Infection Control 32 (February 2004) 12-16.
(13.) Joint Cam mission on Accreditation of Healthcare Organizations, "Surveillance, prevention, and control of infection," in JCAHO Comprehensive Accreditation Manual for Hospitals: The Official Handbook (Oakbrook Terrace, Ill: Joint Commission on Accreditation of Healthcare Organizations, 2004) IC-7.
(14.) Centers for Disease Control and Prevention, "Guidelines for the prevention of intravascular catheter-related infections," Morbidity and Mortality Weekly Report 51 (RR-10) (Aug 9, 2002) 1-29.
15. Hospital Infection Control Practices Advisory Committee, "Part II. Recommendations for Isolation Precautions in Hospitals," Centers for Disease Control and Prevention, http://www.cdc .gov/ncidod/hip/isolat/isopart2.htm (accessed 20 April 2004).
(16.) Communicable Disease Profile--Iraq (news release, Baghdad, Iraq: World Health Organization, March 19, 2003) 39.
(17.) Centers for Disease Control and Prevention, "Cutaneous leishmaniasis in US military personnel--Southwest/Central Asia, 2002-2003," Morbidity and Mortality Weekly Report 52 (Oct 24, 2003) 1009-1012.
Editor's note: At various times throughout the year, the Recommended Practices Committee seeks review and comments on proposed recommended practices from members and other interested individuals. When available, these proposed recommended practices appear on AORN Online at http://www.aorn.org. Interested individuals who do not have access to the Internet may obtain copies of the proposed documents by calling the Center for Nursing Practice, at (800) 755-2676 x 334. Proposed recommended practice documents are available for review and comment for a 30-day period after they are posted. A deadline for comments is indicated with each document. Please check these sources frequently to locate proposed recommended practices. All comments received are considered as the document is finalized. Thank you for your participation.
JOAN BLANCHARD
RN, MSS, CNOR, CIC
PERIOPERATIVE NURSING SPECIALIST
AORN CENTER FOR NURSING PRACTICE
COPYRIGHT 2004 Association of Operating Room Nurses, Inc.
COPYRIGHT 2004 Gale Group