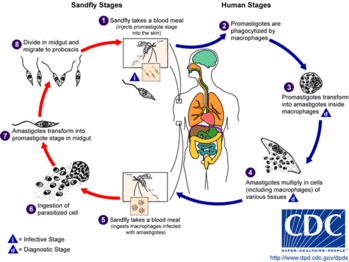
In 2000, a reference center was created to systematically record leishmaniases in Germany. We analyzed 58 cases of leishmaniases imported during a 2-year period. These findings will serve as a baseline for the sandfly vector's anticipated northward move because of global warming and as an advisory for immunocompromised persons traveling to leishmaniasis-endemic areas.
**********
Leishmaniases compose a spectrum of protozoal diseases currently endemic in 88 countries in Asia, Africa, the Americas, and southern Europe. The geographic distribution of leishmaniases has widened, and the disease is reported in areas in which leishmaniasis was previously nonendemic (1). Apart from cutaneous, mucocutaneous/mucosal, and visceral leishmaniases, HIV-1--associated leishmaniasis acquired in southern Europe and in other parts of the world have been observed in increasing numbers (1,2).
Leishmaniasis is not notifiable in Germany. In September 2000, a national advice and reference center was created at the Institute of Tropical Medicine in Berlin; the aim of the center was to monitor the frequency, origin, and type of leishmaniases seen in Germany; to advise physicians; and to improve information for travelers to disease-endemic areas. The healthcare professionals were informed about the reference center by the Robert-Koch-Institute, the center for surveillance of infectious diseases in Germany, as well as through the Journal of the German Medical Association, which is received by every registered physician (3). Leishmaniasis was diagnosed if parasites were detected in smears, culture, histologic sections, by polymerase chain reaction (PCR) of lesion biopsy specimens, bone marrow, or peripheral blood. For detection of Leishmania-specific antigen the small subunit (ssu rRNA), the internal transcribed spacer (ITS-1) region of the ribosomal RNA genes, or both, were amplified by PCR (4). Leishmania complexes and species were determined by digestion of the ribosomal ITS-1 PCR product with restriction enzymes (4).
Within 2 years, 70 cases of leishmaniases (43 cutaneous or mucocutaneous/mucosal; 27 visceral) were reported. For 58 case-patients (35 cutaneous or mucocutaneous/ mucosal; 23 visceral), data were available on the age, sex, residence, travel destination, possible exposure location, reason for travel, duration of stay, duration and type of symptoms, concomitant diseases or therapies, type of diagnosis, and treatment received.
Cutaneous and Mucosal Leishmaniasis
Of the 35 patients with cutaneous or mucocutaneous/ mucosal leishmaniasis, 30 were German tourists (Table 1). The male-to-female ratio was 1.5:1. Ten had contracted cutaneous or mucocutaneous/mucosal leishmaniasis in Europe, 11 in Central and South America, 6 in Asia, and 3 in Africa. Two persons had been infected during work stays of 1 to 4 months in French Guyana, one each in Peru and Libya, and one patient had immigrated from Afghanistan.
The median duration of lesions until the diagnosis of leishmaniasis was made was 4 months (range 3 weeks to 2 years). Sixteen patients had more than one lesion (median 2, range 1-6 lesions). Seventeen lesions were located in the face, including mouth and nose, 28 on the upper extremities and 21 on the lower extremities. Lesions were ulcerated in 39 cases, papular-nodular in 24, and plaque-like in 3. Parasites were detected in 13 of 20 smears, in 9 of 10 cultures, and in 14 of 16 histologic sections; by using PCR, Leishmania-specific DNA was detected in 16 of 16 biopsy specimens.
Patient 1 had lesions in the mouth caused by L. infantum. She was under continuous immunosuppressive treatment for severe bronchial asthma. Patient 2 had received methotrexate and steroids for treatment of systemic collagenosis for several weeks. Both patients were tested for leishmanial infection in the blood; in both patients, the Leishmania-specific PCR of the buffy coat of the blood was positive. Patient 22 had mucocutaneous leishmaniasis of the nasal septum. She had been treated for a skin lesion caused by L. braziliensis 3 years earlier.
Visceral Leishmaniasis
A total of 18 of the 23 visceral leishmaniasis patients were German tourists; 3 were immigrants from Angola, Iran, and Togo; and 2 were visitors from Italy and Portugal (Table 2). The male-to-female ratio was 6.7:1.
The median time between symptom onset and the correct diagnosis was 4 months (range 1-16 months). All case-patients had fever, 17 (74%) had splenomegaly, 11 (48%) hepatomegaly, 20 (87%) anemia, 17 (74%) leukopenia, and 8 (35%) thrombocytopenia.
Bone marrow smears indicated Leishmania in 18 of 20, bone marrow culture in 6 of 7, bone marrow histologic sections in 7 of 8, PCR of the bone marrow in 8 of 9, and PCR of the buffy coat of the blood in 7 of 7 cases. Additionally, antibodies were detected in medium to high concentration by an immunofluorescence test, enzyme-linked immunosorbent assay (ELISA), or both, in 14 of 15 cases. Species was identified in 7 of 18 visceral cases contracted in southern Europe and indicated Leishmania belonging to the L. donovani complex, which implicated infection with L. infantum.
Six cases of visceral leishmaniasis occurred in children 2 months of age to 11 years of age. Four German tourists and two immigrants had long-known HIV infection (median duration 3 years, range 8 months-6 years). All HIV-coinfected patients had CD4-cell counts below 200/[micro]L (median 108, range 23-185 CD4 cells/[micro]L) when the diagnosis of visceral leishmaniasis was made. Of the remaining 11 patients, 1 had a thymoma with impaired T-helper-1 cell function, 2 had received intermittent immunosuppressive therapy (methotrexate and steroids) for rheumatologic disease, and 2 patients had their spleens removed. Three patients were in an impaired general condition because of combinations of diabetes, hypertonus, hypercholesterolemia, and emphysema. In the remaining three patients (53-68 years of age), apart from hypertonus in one, no impairing condition was detected.
Discussion
Information on single cases and a small case series of imported leishmaniases in Germany is available, but systematic reporting on frequency, type, and origin of leishmanial infections in Germany did not exist until 2000 (5-7). Our recent surveillance is dependent on passive consultation and reporting and therefore may have selection bias because if visceral leishmaniasis, a potentially fatal disease that requires hospitalization, is suspected, advice on diagnosis and treatment is sought more often than for the skin infection. We assume that our system captures approximately half of the visceral leishmaniasis cases and approximately one third of the classical cutaneous cases imported to Germany.
A total of 47% of all cases, but 78% of the visceral cases were contracted in the European Mediterranean area and Portugal, and most of the infections indicated a species of the L. donovani complex, most probably L. infantum, as the probable causative agent. Thirteen infections (22%) were acquired on the Mediterranean islands of Ibiza, Ischia, Majorca, Malta, Korfu, or Sicily.
This distribution reflects the fact, that the Mediterranean countries, Spain, Italy, and the Mediterranean islands, in particular, are the favorite vacation areas for Germans. Annually, Germans take 18 million vacations to the European Mediterranean area (including 8 million to Spain and 6 million to Italy) with a median duration of 2 weeks. Sixty percent of travel to Italy and 90% of travel to Spain are to Leishmania-endemic areas.
While leishmaniasis has always been endemic in the Mediterranean countries, the maximum northern latitude for sandfly survival is speculated to move further to the North, beyond Germany (1) because of global warming. If this scenario is correct, the imported cases may serve as a potential substrate for the sandfly vector. Dogs that are imported as pets from the disease-endemic areas of southwestern Europe or that contract the infection when accompanying their owners for vacation are another potential substrate (8).
Infections with L. infantum in a child, as well as in a horse who had never left Germany, have recently been described and have led to speculations about an autochthonous focus (9,10). Also recently, the first sandfly species, Phlebotomus mascittii Grassi, 1908, was detected in southern Germany, although its potential as a vector of Leishmania remains to be demonstrated (11).
As expected, visceral leishmaniasis is often manifested in persons with impaired immunocompetence because of young age, HIV infection, immunosuppressive therapy and, in our analysis, in older persons with concomitant diseases.
Notably, 12 (67%) of 18 of the visceral cases contracted in the European Mediterranean area were in adults, thus confirming a change in age groups affected. Formerly, visceral leishmaniasis was known mainly as a disease of children (1,2). This change may partly be explained by the increased proportion of Leishmania and HIV--co-infected persons and partly by increased travel activities of otherwise immunocompromised persons, including elderly persons.
Furthermore, even in patients with cutaneous leishmaniasis, dissemination of parasites has to be excluded in case of impaired immunocompetence (e.g., immunosuppressive treatment). In these cases, Leishmania-specific PCR of the buffy coat of the peripheral blood is a sensitive method for detecting parasite spread beyond the skin.
Parents of small children and persons with reduced immunocompetence should be informed about their increased susceptibility to infection with Leishmania when traveling to disease-endemic areas. Measures to reduce the exposure to sandflies, such as clothes, repellents, and mosquito nets as well as collars impregnated with repellents for accompanying dogs, should be recommended.
Acknowledgments
We thank colleagues at German hospitals and health institutions who discussed their patients with us or kindly contributed to the data collected on leishmaniases.
References
(1.) Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg 2001;95:239-43.
(2.) World Health Organization. Leishmania/HIV co-infection: southwestern Europe, 1990-1998 (WHO/LEISH/2000.42). Geneva: The Organization; 2000.
(3.) Harms G, Bienzle U. Leishmaniosen-importierte Krankheiten. Dtsch Arztebl 2000;31/32:1589-92.
(4.) Schonian G, Schnur L, Fahri M. Genetic heterogeneity in the species Leishmania tropica revealed by different PCR-based methods. Trans R Soc Trop Med Hyg 2001;95:217-24.
(5.) Hohenschild S, Feldmeier H. Imported kala azar in children and adults--comparison of medical history, clinical and diagnostic findings. J Trop Pediatr 1995;41:378-9.
(6.) Harms G, Zenk J, Martin S, Kokozidou M, Puschel W, Bienzle U, et al. Localized lymphadenopathy due to leishmanial infection. Infection 2001 ;29:355-6.
(7.) Holzer E, Kupferschmidt HG. Cutaneous leishmaniasis in East German citizens. Z Arztl Fortbild (Jena) 1986;80:381-3.
(8.) Gothe R, Nolte I, Kraft W. Leishmaniasis in dogs in Germany: epidemiological case analysis and alternatives to conventional causal therapy. Tierarztl Prax 1997;25:68-73.
(9.) Bogdan C, Schonian G, Banuls AL, Hide M, Pratlong F, Lorenz E, et al. Visceral leishmaniasis in a German child who had never entered a known endemic area: case report and review of the literature. Clin Infect Dis 2001;32:302-6.
(10.) Koehler K, Stechele M, Hetzel U, Domingo M, Schonian G, Zahner H, et al. Cutaneous leishmaniasis in a horse in southern Germany caused by Leishmania infantum. Vet Parasitol 2002;109:9-17.
(11.) Naucke TJ, Pesson B. Presence of Phlebotomus (Transphlebotomus) mascittii Grassi, 1908 (Diptera: Psychodidae) in Germany. Parasitol Res 2000;86:3356.
Address for correspondence: Gundel Harms, Institute of Tropical Medicine Berlin, Spandauer Damm 130, 14050 Berlin, Germany; fax: +49-30-30116-888; email: gundel.harms@charite.de
Gundel Harms, * Gabriele Schonian, * and Hermann Feldmeier ([dagger])
* Charite, Humboldt University Berlin, Berlin, Germany; and ([dagger]) Free University of Berlin, Berlin, Germany
Dr. Harms is a senior lecturer and researcher in tropical medicine and international health at the Institute of Tropical Medicine and at the Medical Faculty Charite, Humboldt University Berlin, Germany. Her primary research interests are the interaction of HIV/AIDS and parasitic infections, leishmaniasis in particular.
COPYRIGHT 2003 U.S. National Center for Infectious Diseases
COPYRIGHT 2003 Gale Group