Abstract
Ivermectin, a broad-spectrum anti-parasitic agent first used in veterinary medicine, is active against numerous specie of helminths and arthropods. For the past few years, world-wide reports on the use of ivermectin in various parasitic diseases with cutaneous tropism have led to its use in the field of skin parasitology. In this paper we review parasitoses which have been treated with ivermectin; mainly, filariasis, strongyloidiasis, cutaneous larva migrans, and scabies and head lice.
Introduction
Ivermectin is a semi-synthetic drug derived from a class of compounds named avermectins. These compounds have an anti-parasitic activity against nematodes and arthropods. This drug has been used extensively for the control of a wide variety of parasites in farm and domestic animals (1-3). In humans, ivermectin has been used extensively to control onchocerciasis in endemic countries of Africa and Latin America since 1987. The drug is also effective in the treatment of other filariases such as loiasis and bancroftian filariasis and other intestinal nematodes (1-4). The interest of dermatologists in ivermectin grew as it became evident that some parasitic infections with cutaneous tropism such as cutaneous larva currens, cutaneous larva migrans and human ectoparasitosis (mainly head lice and scabies) could easily and successfully be treated with the drug given either orally or topically (4,5).
Antiparasitic activity
Avermectins have been isolated in 1979 from the actinomycete Streptomyces avermitilis. Structurally they are macrocyclic lactones similar to the macrolide antibiotics but without antibacterial activity. Ivermectin is a mixture of two chemically modified avermectins (>80% 22,23-dihydroavermectin B1a and <20% of the corresponding B1b-homologue) (1). These compounds induce a paralysis in arthropods and nematodes by interruption of neurotransmission. Ivermectin blocks the chemical transmission across the nerve synapse that use glutamate or g-aminobutyric acid (GABA) (1-3,6-8). The interruption of nerve transmission causes paralysis and death. In mammals, these neurotransmitters and receptors are confined to the central nervous system. In normal conditions the drug does not cross the blood-brain barrier and does not penetrate the central nervous system (1-3,6-8).
Ivermectin resistance has been described for some nematodes and mutations involving glutamate-gated chloride channels a-type subunit have been identified in resistant mutants (85).
Pharmacology
The drug has a good bioavailability with a serum peak 4 hours after an oral dose of 12 mg. The plasma elimination half-life differ varies according to the various metabolites from 12 hours to 3 days. Ivermectin and its metabolites are almost exclusively metabolized in the liever and excreted in the feces (with less than 1% excreted in the urine) (9).
Adverse effects
The safety of the drug has been demonstrated by its extensive use against nematodes. Millions of doses have been distributed in humans worldwide for the treatment of onchocerciasis and other filariasis. Side effects are minor and rare. Most of them have been described in patients treated for filariasis. These include gastrointestinal in 1-2% of treated patients (abdominal pain, anorexia, diarrhea, nausea, vomiting), neurologic, in 1-3% (dizziness, somnolence, vertigo, tremor), cutaneous side-effects in 1-3% (pruritus, rash, urticaria) and rare biological abnormalities 2-3% (elevated transaminases, leukopenia) (2,9). Most side effects occur in the treatment of filariasis due to allergic reactions caused by the death of a great number of microfilariae (10).
Concerns about the safety of ivermectin in elderly patients treated for scabies have not been confirmed (11-15). In some circumstances, toxicity has been reported in some mammals; idiosyncratic toxicity has been observed in collies and herding breeds (7,8,15). It has been suggested that conditions disrupting the blood-brain barrier integrity (such as young mammals or higher dosages) may allow the drug to enter the central nervous system (7,8,15). Therefore, some authors consider the drug contraindicated in children under 5 years of age or under 15 kg, and during pregnancy and lactation. However, there are several reports of the use of ivermectin in children without adverse effect (16,17). The drug is contraindicated in case of allergic sensitizsation and must should be used cautiously in persons who have an infection of the nervous system.
Filariasis
The interest of ivermectin in filariasis has been established since the late Eighties (2). The effect and tolerance of ivermectin is considered to be about the same in all cases of filariasis due to its efficacy on microfilaria, but not adult filaria. Administered as a single dose, ivermectin induces a nearly complete clearance of microfilaremia from blood and skin within thirty days. This is followed by a gradual recurrence of microfilaria and increase in microfilarial load. Higher doses of ivermectin show greater clearance and more prolonged effect. The frequency and intensity of adverse reactions are strongly associated with the pre-treatment microfilarial load, but are independent of the dose of ivermectin.
Onchocerciasis
Mass distribution of ivermectin to individuals at risk of onchocerciasis is the pivotal strategy of three programs whose goal is to eliminate the disease in Africa and the Americas. Ivermectin causes a dramatic decrease of the microfilarial load in the skin of patients with onchocerciasis and thus leads to a clinical improvement of the symptoms (18). Nonetheless, the clinical benefit of one single dose of ivermectin is not very significant. As an example, in one pivotal study, after 6 months such treatment significantly reduced the severity of the disease, particularly the papular eruption, but not the prevalence of skin lesions. There was no reduction in the prevalence of itching, nor improvement in the general health status (19). Indeed, when used as a single annual or semi-annual dose, ivermectin has no effect on the adult female filarial, whose life may last 14 years or longer. Thus to maintain the beneficial aspect of the treatment, ivermectin must be given repeatedly throughout the lifespan of the parasite (20).
The development of new treatment strategies that have a long-lasting effect would be of great benefit to all patients. First it was suggested to repeat cures with ivermectin regularly. It appeared that there was an advantage in terms of antiparasitic effect over a two-year period with treatment given every 6 months compared with yearly administration (21), but this advantage was considered "slight". Recently, two therapeutic options have shown some encouraging results. First, ivermectin combined with long-term treatment with doxycycline may cause the sterilization of the adult female worm, perhaps permanently (22). Secondly, increasing the frequency of ivermectin distribution from annually to every 3 months decreases the fecundity of the adult female parasite two-to-threefold and this effect is long-lasting (23).
There is a lack of severe adverse reactions in the treatment of onchocerciasis in indigenous adult patients, but there are controversies regarding the prevalence of these side effects. In Ghana, mild reactions were reported to occur in 15% of 14,911 persons receiving their first dose of ivermectin; the only severe reaction attributable to ivermectin was severe symptomatic postural hypotension, which was seen in 37 cases (24). In contrast, in Sierra Leone, 32% of 87 patients suffered adverse reactions that required treatment with antihistamines or aspirin (25). In addition, physicians might expect to see a higher frequency of mild or moderate adverse reactions in expatriates with onchocerciasis (26). Of 28 such expatriates living in England, 17 (61%) had reactions to the first dose of ivermectin. All experienced itching and an urticarial eruption, some had localized edema or fever. Symptoms occurred 6 to 36 hours after the dose and continued for 1 to 14 days. Similarly, a high rate (64%) of adverse drug reactions (fever, headache, pruritus, edema, myalgias, arthralgias) has been found in children above 5 years old on the Ivory Coast (27). In adult indigenous patients the incidence of reactions is related to the microfilarial load (24). In contrast, English expatriates had very low microfilarial load (26). It is therefore possible that reactions to ivermectin occur much more frequently in expatriates or children, with recent infections and no immune tolerance, compared to indigenous adults with lifelong exposure and a degree of immune tolerance (26).
Lymphatic filariasis
The efficacy and safety of ivermectin in the treatment of filariasis due to Wuchereria bancrofti has been assessed in a meta analysis of the results front 15 published clinical trials in 1997 (28). There were 7 dose-finding and 8 comparative studies. The findings of the analysis suggest that ivermectin given as a single annual dose of 200 [micro]/kg body weight or higher, with or without in combination with diethylcarbamazine, has great potential as a therapeutic strategy to control bancroftian filariasis. However, there are controversies regarding co-administration of ivermectin with diethylcarbamazine or albendazole. The combination of ivermectin and diethylcarbamazine was significantly more efficient than diethylcarbamazine alone in a large study performed in Papua New Guinea (29). More recently it has been shown in Ghana that the difference in efficacy between the combination of ivermectin and albendazole and ivermectin alone appeared minimal after 12 months, whereas albendazole alone demonstrated significantly decreased efficacy (30). In the treatment of lymphatic filariasis, ivermectin may be used alone or in combination with albendazole or diethylcarbamazine. But no study clearly supports the use of ivermectin-based combinations over ivermectin alone.
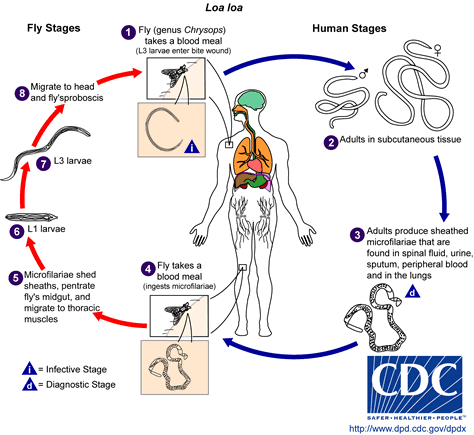
Loiasis
In the treatment of loiasis, ivermectin heavily decreases the microfilaremia and thus more safely allows subsequent treatment with diethylcarbamazine. Nonetheless, ivermectin may also give rise to severe adverse reactions and must be prescribed with caution to patients with high microfilaremia. The potential interest of ivermectin in loiasis has been known since the late eighties. In an open, escalating dose study, 35 patients from Gabon with loiasis received doses of ivermectin varying from 5 [micro]g/kg to 200 [micro]g/kg. Dosages below 50 [micro]g/kg were insufficient. The 200 [micro]g/kg dosage was the most effective, leading to a reduction in the microfilarial load of 88% by day 15 (31). This figure was 92.7% at day 10 in another study involving 7 patients residing in France (32). However the microfilaricidal activity of a single dose of ivermectin appears to be inadequate to cure patients with loiasis. The duration of ivermectin activity and the required frequency of treatment are still unknown. Results of preliminary studies show that the number of annual treatments used in mass therapy in endemic countries can be adapted to provide efficient protection (33).
The treatment of loiasis with ivermectin may produce severe adverse reactions, as diethylcarbamazine, but they are less frequent and are rarely life threatening. Nonetheless, ivermectin may also induce serious neurologic reactions in patients with high microfilaremia (34). In one study in Cameroon, severe adverse reactions occurred in 0.11% of the patients but much more significantly in those with microfilaremia of more than 50,000 mf/ml (35).
Strongyloidiasis
Ivermectin is now the first line of single-dose treatment for strongyloidiasis. Its efficacy and tolerance has been assessed in one dose-ranging study and compared to alternative treatment in two randomized studies. The first escalating dose study included 110 patients with intestinal strongyloidiasis (36). It showed that the cure rates varied according to the daily dosage, from 67% (50 [micro]/kg) to 100% (200 and 400 [micro]/kg). Ivermectin (12 mg single dose, i.e., 150-200 [micro]/kg) was compared to oral albendazole (400 mg/d three consecutive days) in an open randomized comparative trial (37). Sixty patients were included, 53 were evaluable. Tolerance and efficacy were evaluated on day 7, 30, and 90. The criterion of judgement was the negativity of the stool test which always included Baermann technique and was performed at each evaluation. Cure rates were 83% with ivermectin and 38% with albendazole. The difference was statistically significant (p<0.01). Ivermectin (200 [micro]/kg as a single dose on two following days) was also compared to oral thiabendazole (50 mg/kg/day three consecutive days) in one randomised double blinded study (38). Fifty three patients were evaluated in three groups. The differences for cure rates in the three groups were not statistically significant. Nonetheless adverse events were reported by 95% of the patients treated with thiabendazole (nausea, dizziness, fatigue) and 18% of those treated with thiabendazole (pruritis, fatigue, headaches). The difference was statistically significant.
Lastly, ivermectin was effective in the treatment of three cases of larva currens (39). Two patients were treated with one single dose of 12 mg ivermectin and the third (weighing more than 100 kg / 220 lbs) received a second 12 mg dose of ivermectin before cure.
Given these results, ivermectin may be given to patients with larva currens and intestinal strongyloidiasis. Furthermore, it could be recommended for those at risk of strongyloidiasis before receiving an immunosuppressive treatment. The recommended dosage in these indications is 200 [micro]/kg in a single dose. In case of failure the dose must be repeated after one month. In some instances the dosage may be increased to 200 [micro]/kg/day for two consecutive days (400 [micro]/kg).
Cutaneous larva migrans (creeping eruption)
The treatment of choice for cutaneous larva migrans (CLM) is the topical application of a 15% liquid suspension (or ointment) of thiabendazole (41). Nonetheless, topical thiabendazole may be difficult to apply in cases of multiple lesions or lesions located on the soles. Therefore there is place for oral treatment, especially if this treatment is efficacious as a single dose.
Of all the efficient oral antihelminthic agents, ivermectin has the advantage of being well tolerated with high efficacy when taken in a single 12 mg oral dose, the cure rate varying from 77% (42) to more than 94% (43,44). After treatment, the median duration of cessation of pruritus was 3 days (1-7 days) and that of the creeping eruption was 9 days (4-30 days) (43). In this indication, ivermectin has been compared to oral albendazole in a randomized open study. A single 12 mg oral dose of ivermectin was significantly more efficacious than a single 400 mg oral dose of albendazole (100% vs. 46%; p=0.017) (45). If pruritus persists or if the larva is still migrating after one week, a second or a third course of ivermectin, one week apart, may be necessary.
The experience with ivermectin in the treatment of hookworm folliculitis is different. The clinical response to a single 12 mg dose of oral ivermectin was less favorable in our patients with folliculitis than in most other cases of CLM. In addition, in one study of five patients with hookworm folliculitis and CLM, only two were cured with ivermectin (unit dose 12 mg) after one course whereas two courses one week apart were necessary in 2 cases, and three courses one week apart in 1 case (46). Treatment was repeated until the pruritus disappeared, this symptom being indicative of parasite viability. Lesions of folliculitis took longer to clear up than those of creeping eruptions.
Gnathostomiasis
It has been recently suggested that ivermectin should also be an effective treatment of gnathostomiasis (47,48). In one study, a single dose of 0.2 mg/kg of ivermectin gave a 95% cure rate in 21 Thai patients (48). However, these reports lack any rate of follow up (no more than six months), and in endemic countries it may be difficult to distinguish relapses from reinfections. A small series (but of imported cases with prolonged period of follow up without possibility of reinfections) suggests that treatment with ivermectin is not as efficient in tourists returning from Southeast Asian countries than it is in Thai people.
Medical treatment of gnathostomiasis may also lead to the migration of Gnathostoma larvae superficially, so that they could be removed from the skin. It has been observed in 5% of the patients treated with ivermectin, and 6% of the patients treated with albendazole in a recent comparative series (48).
Toxocariasis
Ivermectin did not show a significant efficacy in an open study of visceral larva migrans related to infection with Toxocara canis or T. catis (51).
Scabies
Ivermectin in common scabies
Six comparative trials have been reported with the use of oral ivermectin in the treatment of ordinary scabies. Macotela-Ruiz et al. (52) described a double blind trial which compared ivermectin 200 [micro]g/kg, single dose versus placebo. Of the 55 patients included, 26 of 29 (79%) were considered cured at the first visit at day 7 in the ivermectin group versus 4 over 26 (16%) in the placebo group (p<0.001). Glaziou et al. (53) described an investigator-blinded trial involving 44 subjects, which compared a single oral dose of ivermectin 100mg/kg with a topical treatment, benzyl benzoate 10%. At day 30, 16 of 22 (70%) in the ivermectin group versus 10 of 21 (48%) were cured, but the difference was not significant. Chouela et al. (54) compared lindane 1% solution versus 150-200 [micro]g/kg ivermectin in a randomized double blind study. Fifty-three patients were included, 43 completed the study. At day 15, 14 patients (74%) treated with ivermectin were healed versus 13 (54%) in the lindane group. At day 29, after a repeated treatment for patients not cured, 18 patients (95%) were healed in the ivermectin group versus 23 (96%) in the lindane group. Both treatments were considered of equivalence efficacy at day 29. In a recent comparative study, ivermectin had better results than lindane lotion 1% (55). Usha et al. [56] described an open randomized study comparing oral ivermectin 200 [micro]g/kg with 5% permethrin cream. By the first and second week respectively, 20 (50%) and 28 (70%) in the ivermectin group were cured versus 35 (84.5%) and 44 (97.8%) in the permethrin group. After a second dose, by the fourth week 38 (95%) of the patients in the ivermectin group were cured. A recent trial compared oral ivermectin 200 [micro]g/kg single dose with topical benzyl benzoate 10%. One hundred ten children aged 6 months to 14 years were treated, and there was no difference between the two treatments for efficacy at 3 weeks respectively and no serious side effects, but benzyl benzoate produced more skin irritation (17). Only one report fails to show any efficacy of the drug (57).
The drug was also active in nodular scabies showing gradual improvement in a few reports (58,59). A transitory aggravation of pruritus is possible the first days after treatment and must not be considered as a treatment failure (56,60-64). A few open studies involving 50, 29 and 10 patients reported the efficacy of topical ivermectin formulations (65-67).
Ivermectin in crusted scabies and immuno-compromised patients
Different schemes have been used in the treatment of crusted scabies: ivermectin alone (in a single dose or repeated doses), or in conjunction with keratolytic agents, or topical scabicidal agents (61,68-76). In some cases a cure was achieved in crusted scabies that had been refractory to topical conventional scabicides (77). The concomitant use of topical scabicides and keratolytics improves the ability of the drug to penetrate the crust and hyperkeratotic scale (69,74). Huffam et al. (74) observed a complete response in 8 out of 20 patients with Norwegian scabies treated with oral ivermectin 200mg/kg combined with topical permethrin 5%; however, recurrence or re-infestations occurred in half of them. In vitro studies showed that these relapses were not related to drug resistance (76).
In HIV-infected patients for whom scabies is more difficult to treat, Meinking et al. (69) reported therapeutic success in patients with common scabies. Alberici et al. (78) treated 60 cases of common or crusted scabies in 39 HIV-infected patients, and obtained an optimal rate of success with the combination of topical scabicide with oral ivermectin.
Ivermectin in institutions
Outbreaks of scabies in institutions have traditionally been difficult to eradicate. Ivermectin has shown great promise in the treatment of such revised cases. Marty et al. (60) eradicated scabies in a geriatric nursing home by the generalized distribution of the drug to the staff members and residents. Several other reports confirmed the ability of the oral formulation to eradicate scabies in different epidemic or endemic situations in nursing homes (6,11,12,79,80). Leppard et al. (81) were able to control an outbreak of scabies in a prison in Tanzania by giving a single dose of 150 [micro]g/kg under supervision to 1153 prisoners. The drug can be useful in health public programs. In Papua, New Guinea the mass treatment of lymphatic filariasis with ivermectin resulted in a marked reduction of scabies in the population (82). Topical permethrin, oral ivermectin, and improved hygiene measures led to the control of scabies in rural community in an Egyptian village (83).
Ivermectin as a systemic alternative to topical scabicides
Ivermectin offers an alternative therapy for the treatment of scabies (84). In France, considering the utility of the drug, approval was given the drug is licensed for human scabies since in October 2001. The treatment is easy, quick, safe and well tolerated, with maximal patient compliance. More studies are needed to define the optimal dosing for patients with scabies. Most authors used 200 [micro]g/kg per daily dose, yet to date, no study has been done to compare the 200 [micro]g/kg dose with other doses used in previous studies. In common scabies, a single dose is usually enough to cure the disease. In crusted scabies or in institutional epidemics, repeated doses may be necessary seems to be required to achieve maximal efficacy. Two doses of ivermectin were found to be 100% effective when used to treat ordinary scabies. The failure of a single dose relates to the lack of ovicidal action of the drug. One additional dose seems indicated but comparative studies are lacking. Considering both the half-life elimination of the drug and the delay of eggs hatching, the appropriate delay between doses is considered as one or two weeks, but here comparative studies are lacking as well. The value of combined treatment with topical treatment in crusted scabies also needs to be addressed, as well as using ivermectin for the treatment of asymptomatic family members or hospital or nursing home staff members in case of outbreaks. Although measures such as cleaning of clothes and bed linens are recommended, the consequence of the use of oral ivermectin on these measures has not been studieds. The emergence of scabicidal resistance to ivermectin also needs to be addressed.
Pediculosis
A few studies have reported the efficacy of oral or topical formulations in the treatment of pediculosis. Dunne et al. (55) found that a single oral dose of 100-200 [micro]g/kg ivermectin had a significant effect in reducing head lice infestation when compared with controls. Glaziou et al. (86) treated 26 patients with a single 200 [micro]/kg oral dose of ivermectin. At day 14 after treatment, 20 responded to the treatment (77%), and 6 patients (23%) presented with a complete disappearance of eggs and all clinical symptoms. At day 28, some healed patients had signs of reinfestation. Therefore, the authors suggested giving a second dose at day 10. Topical ivermectin has also been used with success. Youssef et al. (87) reported that a single application of 0.8% ivermectin lotion was efficient to treat head lice in all 25 patients treated with clearance of parasites within 48 hours after application. A 0.8% shampoo was more effective than a shampoo containing 1% g-benzene hexachloride in 208 Columbian patients (88). Even though more clinical trials are needed to confirm this efficacy, ivermectin provides a promising alternative therapy for head lice, particularly in a period of increasing resistance to current available topical treatments (89).
Other Arthropods
Demodicidosis
Demodex mites are common commensals of the pilosebaceous unit in mammals (91). In dogs, demodicidosis is a severe disease with massive infestation. Ivermectin and derivates are extremely efficient treatment in these animals. In human the pathogenicity of the two human species (Demodex brevis and Demodex folliculorum) is controversial. Given Tthe efficacy of ivermectin against animal Demodex, one can suggest that the drug can be used against human mites. Only a single case of a rosacea-like demodicidosis successfully treated with oral ivermectin and topical permethrin and 2 cases of papulo-pustular eruption in AIDS patients successfully treated with oral ivermectin have been reported (92,93).
Myiases
Since ivermectin is active against animal myiasis, it is likely that the drug is also active against human myiasis. A few reports confirm this view. Victoria et al. (94) in Colombia and Clyti et al. (95) in French Guyana have successfully treated 4 and 5 patients with Cochliomyia hominivorax with topical ivermectin. Jelinek used oral ivermectin for a cutaneous myiasis with Hypoderma lineatum (96).
Conclusion
Ivermectin should be considered as a new weapon in the field of parasitic dermatology. The drug is easy to use, safe, and has convenient dosing which improves compliance. It has shown efficacy in numerous diseases, although randomized clinical trials are sometimes lacking. As for onchocerciasis, health public programs should be established in developing countries to control cutaneous parasitic diseases that may be associated with significant morbidity. Indeed, ivermectin may replace the use of topical treatments for many parasitic infections and diseases worldwide.
References
(1.) Jackson HC. Ivermectin as a systemic insecticide. Parasitol Today 1989; 5:146-56.
(2.) Ottesen EA, Cambell WC. Ivermectin in human medicine. J Antimicrob Chemother 1994; 195-203.
(3.) Wilson ML. Avermectins in arthropods vector management-prospects and pitfalls. Parasitol Today 1993; 9:84-7.
(4.) Caumes E. Ivermectine et dermatoses tropicales. Bull Soc Pathol Exot 1997; 90:37-8.
(5.) Del Giudice P, Marty P. Ivermectin: a new therapeutic weapon in dermatology? Arch Dermatol 1999; 135:705-6.
(6.) Paasch U, Haustein UF. Management of endemic outbreaks of scabies with allethrin, permethrin, and ivermectin. Int J Dermatol 2000; 39:463-70.
(7.) Burkhart CN, Burkhart CG. Before using ivermectin therapy for scabies. Pediatr Dermatol 1999; 16:478-80.
(8.) Burkhart CN, Burkhart CG. Ivermectin: a few caveats are warranted before initiating therapy for scabies. Arch Dermatol 1999; 135:1549-50.
(9.) Goa KL, McTavish D, Clissold SP. Ivermectin. A review of its antifilarial activity, pharmacokinetic properties and clinical efficacy in onchocerciasis. Drugs. 1991; 42:640-58.
(10.) Gardon J, Gardon-Wendel, Demanga-Ngangue, Kmagno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997; 350:18-22.
(11.) Barkwell R, Shiels S. Deaths associated with ivermectin treatment of scabies. Lancet 1997; 349:1144-5.
(12.) Vasseur E, Fleury L, Bougnoux ME, Moussally S, Vilmur V, Cassou B, Brucker G, Saiagd P. Evaluation de l'efficacite de l'ivermectine au cours d'une epidemie de gale en hopital de long sejour. Journees Dermatologiques de Paris 1998; 2-5 December 1998; Paris. Ann Dermatol Venereol 1998; 125(suppl 3):202.
(13.) Del Giudice P, Marty P, Toussaint-Gari M, Le Fichoux Y. Ivermectin in elderly patients. Arch Dermatol 1999; 135:351-2.
(14.) Alexander NDE, Bockarie MJ, Kastens WA, Karuza JW, Alpers MP. Absence of ivermectin-associated excess deaths. Trans R Soc Trop Med Hyg 1998; 92:342.
(15.) Bredal WP. Deaths associated with ivermectin for scabies. Lancet 1997; 350:216:7.
(16.) Del Mar Saez-de-Ocariz M, McKinster CD, Orozco-Covarrubias L, Tamayo-Sanchez L, Ruiz-Maldonado R. Treatment of 18 children with scabies or cutaneous larva migrans using ivermectin. Clin Exp Dermatol 2002; 27:264-7.
(17.) Brooks P, Grace R. Ivermectin is better than benzyl benzoate for childhood scabies in developing countries. J Paediatr Child Health 2002; 38:401-4.
(18.) Newland HS, White AT, Greene BM et al. Effect of single dose ivermectin therapy on human Onchocerca volvulus infection with onchocercal ocular involvement. Br J Ophthalmol 1998. 72:561-569.
(19.) Whithworth JA, Morgan D, Maude GH, Downham MD, Taylor DW. A community trial of ivermectin for onchocerciasis in Sierra Leone: clinical and parasitological responses to the initial dose. Trans R Soc Trop Med Hyg 1991; 85:92-96.
(20.) Unnasch TR. River blindness. Lancet 2002; 360:182-183.
(21.) Greene BM, Dukuly ZD, Munoz B, White AT, Pacque M, Taylor HR. A comparison of 6-, 12-, and 24-monthly dosing with ivermectin for treatment of onchocerciasis. J infect Dis 1991; 163:376-380.
(22.) Hoerauf A, Mand S, Adjei O, Fleischer B, Buttner DW. Depletion of wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet 2001; 357:1415-1416.
(23.) Gardon J, Boussinesq M, Ka mgno J, Gardon-Wendel N, Demanga-Ngangue J, Duke BO. Effects of standard and high doses ofivermectin on adult worms of Onchocerca volvulus: a randomised controlled trial. Lancet 2002; 360:203-210.
(24.) De Sole G, Awaddzi K, Remme J, et al. A community trial of ivermectin in the onchocerciasis focus of Asubende, Ghana II. Adverse reactions. Trop Med Parasitol 1989; 40:375-382.
(25.) Rothova A, van der Lelij, Stilma JS, Wilson WR, Barbe RF. Side-effects of ivermectin in treatment of onchocerciasis. Lancet 1989; i:1439-1441.
(26.) Davidson RN, Godfrey-Fausset P, Bryceson AD. Adverse reactions in expatriates treated with ivermectin. Lancet 1990; 336:1005.
(27.) Lariviere M, Beauvais B, Aziz M et al. Etude en Cote d'Ivoire (1985-1987) de l'efficacite et de la tolerance de l'ivermectine (Mectizan) dans l'onchocercose humaine III. Tolerance et efficacite d'une seule dose orale de 150 [micro]/kg chez des enfants. Bull Soc Ppath Exot 1989; 82:58-64.
(28.) Cao WC, Van der Ploeg CP, Plaisier AP, van der Sluijs IJ, Habbema JD. Ivermectin for the chemotherapy of bancroftian filariasis: a meta-analysis of the effect of single treatment. Trop Med Int Health 1997; 2:393-403.
(29.) Bockarie MJ, Alexander ND, Hyun Pet al. Randomised community based-trial of annual single-dose diethylcarbamazine with or without ivermectin against Wuchereria bancrofti infection in human beings and mosquitoes. Lancet 1998;351:162-168.
(30.) Dunyo SK, Nkrumah FK, Simonsen PE. Single-dose treatment of Wuchereria bancrofti infections with ivermectin and albendazole alone or in combination: evaluation of the potential for control at 12 months after treatment. Trans R Soc Trop Med Hyg 2000;94(4):437-443.
(31.) Richard-Lenoble D, Kombila M, Rupp EA, et al. Ivermectin in loiasis and concomitant O. Volvulus and M. perstans infections. Am J Trop Med Hyg 1988;39:480-483.
(32.) Paris L, Datry A, Durepaire R, Felix H, Gaxotte P, Danis M et al. Interet de l'ivermectine dans le traitement initial de la loase. Presse Med 1991;20:1393.
(33.) Kombila M, Duong TH, Ferrer A, Perret JC, Marion MC, Nguiri C et al. Short and long-term action of multiple doses of ivermectin on loiasis microfilaremia. Am J trop Med Hyg 1998;58:458-460.
(34.) Boussinesq M, Gardon J, Gardon-Wendel N, Ka mgno J, Ngoumou P, Chippaux JP. Three probable cases of Loa loa encephalopathy following ivermectin treatment for onchocerciasis. Am J Trop Med Hyg 1998;58:461-469.
(35.) Gardon J, Gardon-Wendel N, Demanga-Ngangue, Kmagno J, Chippaux JP, Boussinesq M. Serious reactions after mass tretament of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997;350:18-22.
(36.) Naquira C, Jimenez G, Guerra JG, Bernal R, Nalin DR, Neu D et al. Ivermectin for human strongyloidiasis and other intestinal helminths. Am J Trop Med Hyg 1989; 40:304-309.
(37.) Datry A, Hilmarsdottir I, Mayorga-Sagastume R, Lyagoubi M, Gaxotte P, Biligui S et al. Treatment of Strongyloides stercoralis infection with ivermectin compared with albendazole: results of an open study of 60 cases. Trans R Soc Trop Med Hyg 1994;88:344-345.
(38.) Gann P.H., Neva F.A., Gam A.A. A randomized trial of single- and two-dose ivermectin versus thiabendazole for treatment of strongyloidiasis. J Infect Dis 1994;169:1076-1079.
(39.) Caumes E, Datry A, Mayorga R, Gaxotte P, Danis M, Gentilini M. Efficacy of ivermectin in the therapy of larva currens. Arch Dermatol 1994;130:932.
(40.) Freedman DO, Zierdt WS, Lujan A, Nutman TB. The efficacy of ivermectin in the chemotherapy of gastrointestinal helminthiasis in humans. J Inf Dis 1989;159:1151-1153.
(41.) Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis 2000; 30:811-814.
(42.) Bouchaud O, Houze S, Schiemann R, et al. Cutaneous larva migrans in travellers: a prospective study, with assessment of therapy with Ivermectin. Clin Infect Dis 2000; 31:493-498.
(43.) Caumes E, Carriere J, Guermonprez G, Bricaire F, Danis M, Gentilini M. Dermatoses associated with travel to tropical countries: a prospective study of the diagnosis and management of 269 patients presenting to a tropical disease unit. Clin Infect Dis 1995:20:542-548.
(44.) Van Den Enden E, Stevens A, Gompel AV. Treatment of cutaneous larva migrans. N Engl J Med 1998; 339:1246-1247.
(45.) Caumes E, Carriere J, Datry A, Danis M, Gentilini M. A randomized trial of ivermectin versus albendazole for the treatment of cutaneous larva migrans. Am J Trop Med Hyg 1993;49:641-644.
(46.) Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol 2002; 146:1-3.
(47.) Chappuis F, Farinelli T, Loutan L. Ivermectin treatment of a traveler who returned from Peru with cutaneous gnathostomiasis. Clin Infect Dis, 2001; 33:e17-19.
(48.) Nontasut P, Bussaratid V, Chullawichit S, Charoensook N, Visetsuk K. Comparison of ivermectin and albendazole treatment for gnathostomiasis. Southeast Asian J Trop Med Public Health 2000; 31:374-377.
(49.) Menard A, Dos Santos G, Dekumyoy P et al. Imported cutaneous gnathostomiasis: report of 5 cases. Trans R Soc Trop Med Hyg 2002, in press.
(50.) Kraivichian P, Kulkumthorn M, Yingyourd P, Akarabovorn P, Paireepai C. Albendazole for the treatment of human gnathostomiasis. Trans R Soc Trop Med Hyg 1992; 86:418-421.
(51.) Magnaval JF. Apparent weak efficacy of ivermectin for the treatment of human toxocariasis. Antimicrob Agents Chemother 1998; 42:2270.
(52.) Macotela-Ruiz, Pena-Gonzalez G. Tratamiento de la escabiasis con ivermecia pot via oral. Gaceta Medica de Mexico 1993; 129:201-5.
(53.) Glaziou P, Nguyen LN, Moulia-Pelat JP, Cartel JL, Martin PMV. Efficacy of ivermectin for the treatment of head lice (Ppediculosis capitis). Trop Med Parasitol 1994; 45:253-4.
(54.) Chouela, EN, Abeldano AM, Pellerano G, La Forgia M, Papale RM, Garsd A, Del Carmen Balian M, Battista V, Poggio N. Le titre Arch Dermatol 1999; 135:651-655.
(55.) Madan V, Jaskiran K, Gupta U, Gupta DK. Oral ivermectin in scabies patients: a compartison with 1% topical lindanee lotion. J Ddermatol 2001; 28:481-484.
(56.) Usha V, Golopalakrishnan TV. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. J Am Acad Dermatol 2000; 42:236-240.
(57.) Dunne CL, Malone CJ, Whitworth JAG. A field study of the effects of ivermectin on ectoparasites of man. Trans R Soc Trop Med Hyg 1991; 85:550-551.
(58.) Offidiani A, Cellini A, Simonetti O, Fumelli C. Treatment of scabies with ivermectin. Eur J Dermatol 1999; 9:100-1.
(59.) Bauer J, Blum A, Sonnichsen K, Metzler G, Rassner G, Garbe C. Nodular scabies detected by computed dermatoscopy. Dermatology 2001; 203:190-191.
(60.) Marty P, Gari-Toussaint M, Le Fichoux Y, Gaxotte P. Efficacy of ivermectin in the treatment of an epidemic of sarcoptic scabies. Ann Trop Med Parasitol 1994; 88:453.
(61.) Del Giudice P, Carles M, Couppie P, Bernard E, Lacour JP, Marty P, Pradinaud R, Ortonne JP, Dellamonica P, Le Fichoux Y. Successful treatment of crusted (Norwegian) scabies with ivermectin in two patients with human immunodeficiency virus infection. Br J Dermatol 1996; 135:494-495.
(62.) Patel A, Hogan P, Walder B. Crusted scabies in two immuno-compromised children: successful treatment with oral ivermectin. Aust J Dermatol 1999; 40:37-40.
(63.) Dourmishev A, Serafimova D, Dourmishev L. Efficacy and tolerance of oral ivermectin in scabies. J Eur Acad Dermatol Venereol 1998; 11:247-251.
(64.) Burkhart CG, Burkhart CN, Burkhart KM. An epidemiologic and therapeutic reassessment of scabies. Cutis 2000; 65:233-40.
(65.) Victoria J, Trujillo R. Topical ivermectin: a new successful treatment for scabies. Pediatr Dermatol 2001; 18:63-65.
(66.) Yousseff MY, Sadaka HA, Eissa MM, el-Ariny AF. Topical application of ivermectin for human ectoparasites. Am J Trop Med Hyg 1995; 53:652-653.
(67.) Yeruham I, Hadani A. Control of human scabies by topical application of ivermectin. Ann Trop Med Parasitol 1998; 92:627-629.
(68.) Aubin F, Humbert P. Ivermectin for crusted (Norwegian) scabies. N Engl J Med 1995; 332:612.
(69.) Meinking TL, Taplin D, Hermida JL, Pardo R, Kerdel F. The treatment of scabies with ivermectin. N Engl J Med 1995; 333:26-30.
(70.) Corbett EL, Crossley I, Holton J, Levell N, Miller R, De Cock KM. Crusted ("Norwegian") scabies in a specialist HIV unit: successful use of ivermectin and failure to prevent nosocomial transmission. Genitourin Med 1996; 72:115-117.
(71.) Pellizzer G, Betto P, Benedetti P, De Lalla F. Ivermectin treatment of AIDS-related, crusted scabies. Eur J Dermatol 1996; 6:396.
(72.) Cordoliani F, Vasseur E, Baccard M, Fournier S, Feuilhade de Chauvin M, Tancrede E, Morel P. Ivermectin-responsive crusted scabies in HTLV-1 carrier. Dermatology 1996; 192:351-352.
(73.) Dourmishev AL, Serafimova DK, Dourmishev LA, Mualla MA, Papaharalambous V, Malchevsky T. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin, Int J Dermatol 1998; 37:231-4.
(74.) Huffam SE, Currie BJ. Ivermectin for sarcoptes scabiei hyperinfestation. Int J Infect Dis 1998; 2:152-4.
(75.) Ndiaye, B, Develoux M, Dieng MT. Crested (Norwegian) scabies in Dakar (Senegal). Sante 1999; 9:243-248.
(76.) Walton SF, Myerscough MR, Currie BJ. Studies in vitro on the relative efficacy of current acaricides for Sarcoptes scabiei var. hominis. Trans R Soc Trop Med Hyg 2000; 94:92-96.
(77.) Jaramillo-Ayerbe F, Berrio-Munoz J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol 1998; 134:143-5.
(78.) Alberici F, Pagani L, Ratti G, Viale P. Ivermectin alone or in combination with benzyl benzoate in the treatment of human immunodeficiency virus-associated scabies. Br J Dermatol 2000; 142:969-72.
(79.) Reintjes R, Hoek C. Deaths associated with ivermectin for scabies. Lancet 1997; 350:215.
(80.) Sullivan JR, Watt G, Barker B. Successful use of ivermectin in the treatment of endemic scabies in a nursing home. Australas ???? J Dermatol 1997; 38:137-140.
(81.) Leppard B, Naburi AE. The use of ivermectin in controlling an outbreak of scabies in a prison. Br J Dermatol 2000; 143:520-3.
(82.) Bockarie MJ, Alexander NDE, Karuza JW, Bockarie F, Griffin L, Alpers MP. Treatment with ivermectin reduces the high prevalence of scabies in a village in Papua New Guinea. Acta Tropica 2000; 75:127-30.
(83.) Hegazy AA, Drawish NM, Abdel-Hamid IA, Hammad SM. Epidemiology and control of scabies in an Egyptian village. Int J Dermatol 1999; 38:291-6.
(84.) Del Giudice P. Ivermectin in scabies. Current Opin Infect Dis 2002; 15:123-6.
(85.) Dent JA, Smith MM, Vassilatis DK, Avery L. The genetics of ivermectin resistance in Caenorhabditis elegans. ProcROC Natl Acad USA 2000; 97:2674-79.
(86.) Glaziou P, Nyguyen LN, Moulia-Pelat JP, Cartel JL, Martin PM. Efficacy of ivermectin for the treatment of head lice (Pediculosis capitis). Trop Med Parasitol 1994; 45:253-4
(87.) Yousseff MY, Sadaka HA, Eissa MM, el-Ariny AF. Topical application of ivermectin for human ectoparasites. Am J Trop Med Hyg 1995; 53:652-3.
(88.) Halpert. Comparative study of ivermectin and gamma benzene hexachloride in shampoo for the treatment of pediculosis capitis. 8th International Congress Pediatric Dermatology, Paris, 1998, O 18.
(89.) Chosidow O. Scabies and pediculosis. Lancet 2000; 355:819-26.
(90.) Mumcuoglu KY, Miller J, Rosen LJ, Galun R. Systemic activity of ivermectin on the human body louse (Anoplura: Pediculidae). J Med Entomol 1990; 27:72-5.
(91.) Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol 2002; 82:3-6.
(92.) Forstinger C, Kittler H, Binder M. Treatment of rosacea-like demodicidosis with oral ivermectin and topical permethrin cream. J Am Acad Dermatol 1999; 41:775-7.
(93.) Clyti E, Couppie P, Sainte-Marie D, Grosshans E, Pradinaud R. Demodecie et infection par le VIH. Congres de l'Association des Dermatologiste de Langue Francaise, Cayenne February 2000, French Guiana. Ann Dermatol Venereol 2000; 127:AF11.
(94.) Victoria J, Trujillo R, Barreto M. Myiasis: a medical successful treatment with topical ivermectin. Int J Dermatol 1999; 38:142-44.
(95.) Clyti E, Mahdoui C, Couppie P, Fouque F, Cazanave C, Sainte-Marie D, Pradinaud R. Traitement des myiases a Cochliomyia hominivorax par application locale d'ivermectine. Congres de l'Association des Dermatologiste de Langue Francaise, Cayenne February 2000, French Guiana. Ann Dermatol Venereol 2000; 127:SAF13.
(96.) Jelinek T, Northdurft, Rieder N, Locher T. Cutaneous myiasis: review of 13 cases in travellers returning from tropical countries. Int J Dermatol 1995; 34:624-6.
ADDRESS FOR CORRESPONDENCE:
Pascal del Giudice
Unit Infectiologie-Dermatologie
Hopital Bonnet
83600, Frejus
France
E-mail: del-giudice-p@chi-frejus-saint-raphael.fr
PASCAL DEL GIUDICE MD (1), OLIVIER CHOSIDOW, MD, PHD (2), ERIC CAUMES, MD (3).
(1.) PASCAL DEL GIUDICE, UNITE INFECTIOLOGIE-DERMATOLOGIE, HOPITAL BONNET, 83600, FREJUS, FRANCE
(2.) SERVICE DE MEDECINE INTERNE, HOPITAL PITIE-SALPETRIERE, PARIS, FRANCE
(3.) SERVICE DE MALADIES INFECTIEUSES, HOPITAL PITIE-SALPETRIERE, PARIS, FRANCE
COPYRIGHT 2003 Journal of Drugs in Dermatology
COPYRIGHT 2003 Gale Group