A new therapy to slow vision loss in people with wet age-related macular degeneration (AMD), an eye disease that destroys central vision, has been approved by the FDA.
Macugen (pegaptanib sodium injection) is the first in a new class of ophthalmic drugs to specifically target vascular endothelial growth factor (VEGF), a protein that acts as a signal in triggering the abnormal blood vessel growth and leakage that is the hallmark of wet AMD.
AMD is the leading cause of irreversible blindness in patients older than 50 in developed countries. According to a study in the April 2004 issue of Archives of Ophthalmology, AMD is the leading cause of blindness for white Americans, with 1.8 million cases reported in 2002.
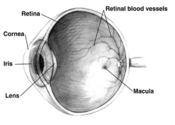
The disease occurs in two forms: wet and dry. Wet AMD occurs when abnormal blood vessels behind the retina start to grow under the macula--located in the center of the retina. These new blood vessels tend to be fragile and often leak blood and fluid. The blood and fluid raise the macula from its normal place at the back of the eye, causing rapid damage to occur. An early symptom of wet AMD is straight lines that appear wavy.
Dry AMD occurs when the light-sensitive cells in the macula slowly break down, gradually blurring central vision in the affected eye. The most common symptom of this type is slightly blurred vision. Wet AMD, which makes up about 10 percent of AMD cases, is considered to be more severe than the dry form.
Approved for marketing in December 2004, Macugen is a single strand of nucleic acid that specifically binds to a particular protein that plays a critical role in the formation of new blood vessels and increased leakage from blood vessels--two of the primary pathological processes responsible for the vision loss associated with wet AMD.
Macugen therapy was co-developed by Eyetech Pharmaceuticals Inc. and Pfizer Inc., both of New York City.
COPYRIGHT 2005 U.S. Government Printing Office
COPYRIGHT 2005 Gale Group