Abstract
Dioxin toxicity can lead to severe cutaneous and systemic disease. The poisoning of Ukrainian President Viktor Yushchenko dramatically illustrates the damage dioxin can inflict. The hallmark of dioxin toxicity is chloracne and should alert the clinician to this diagnosis, which can be confirmed by blood tests. Olestra, a non-absorbable, non-digestible fat substitute is a promising new treatment to lower systemic dioxin levels.
**********
Introduction
In early September 2004, Ukrainian presidential candidate Viktor Yushchenko believed he was poisoned at a dinner party. About 3 hours after dinner, he complained of a headache and the next day developed an acute stomach ache. Five days later, he arrived at the hospital unable to walk. Blood tests revealed a high white blood cell count and elevated liver enzymes. Scans showed that the liver, pancreas, and intestines were swollen. Endoscopy showed multiple intestinal ulcerations.
Tests for common poisons were negative and Yushchenko gradually recovered and returned to the campaign trail. Political opponents attributed the illness to bad sushi or too much alcohol.
Soon, however, he developed acneiform lesions on the face and trunk. He also suffered from severe back pain requiring epidural anesthesia. Gradually, over the next month, the skin lesions worsened to confluent cysts and nodules--hallmarks of chloracne (Figure 1). Blood samples were taken showing 100,000 units per gram of blood fat of the most harmful known dioxin, TCDD--the second highest concentration in a human on record.
Despite the disfigurement caused by dioxin poisoning, Yushchenko won the presidential election. The long-term internal effects of dioxin poisoning of this severity are unknown. He will suffer from chloracne for years, or even decades.
Dioxins
Dioxins are byproducts of manufacturing processes that utilize chlorine, such as in the manufacturing of herbicides, paper, and pulp bleaching. Waste incinerators also produce dioxin as a byproduct. As a result of its wide use in manufacturing and the contamination of soil and water, most people in the United States and Europe have small amounts of a mix of several dioxins in their bodies (15-45 units per gram of blood fat).
Of the hundreds of dioxins, TCDD is the most harmful. TCDD is chemically known as 2,3,7,8-tetrachlorodibenzoparadioxin and was the main component in Agent Orange. Agent Orange was an herbicide used during the Vietnam War to clear vegetation and expose the enemy. The herbicide became well known after the war for the myriad of health problems attributed to its use.
TCDD currently serves no commercial purpose. Pure TCDD must be manufactured in a laboratory. It is made in labs in Europe, Russia, and the United States for use as a control for measuring dioxin levels.
The fact that pure TCDD was found in Ukrainian President Viktor Yushchenko's blood suggests that he was poisoned.
Health Effects of Dioxin
In large doses, dioxin is one of the most toxic synthetic compounds. At a similar dose, TCDD is more toxic than cyanide, cantharidin, nicotine, or colchicine. (1)
The most common acute adverse effect of TCDD exposure is liver damage resembling viral hepatitis. Conjunctivitis, hypertriglyceridemia, prolonged protime, thrombocytopenia, diarrhea, porphyria cutanea tarda, hyperpigmentation, hirsutism, polyneuropathies, and depression may also occur. (2)
The long-term health effects of dioxin poisoning in humans have not been conclusively proven. After the Vietnam War, many alleged that dioxin was the cause of malignant tumors, sterility, abortion, and birth defects. However, no clearly defined mutagenic effects have been demonstrated in humans. Only in doses that cause considerable systemic toxicity has TCDD been shown to cause cancer in rats. (1)
[FIGURE 1A OMITTED]
The most extensive experience with TCDD in humans involves the residents of Seveso, Italy. In 1976, TCDD was released in a plume from a chemical plant when a safety disk ruptured. More than 37,000 persons were exposed to varying amounts of TCDD. Except for chloracne, no significant long-term health problems or pathologic laboratory parameters were found after 20 years of observation. (3)
Chloracne
Chloracne is the hallmark of dioxin toxicity. The term was introduced in 1899 by Herxheimer who believed this acneiform eruption was due to chlorine gas involved in the production of herbicides and wood preservatives. (4) Although some individuals seem to be resistant, the great majority of patients exposed to toxic doses of dioxin will exhibit chloracne.
[FIGURE 1B OMITTED]
The clinical course of chloracne varies. Usually 2 to 4 weeks after initial exposure, the patient will develop open comedones evolving into noninflammatory yellow cysts. These cysts appear on the malar cheeks, lateral to the eyes, and behind the ears. With more severe or chronic exposure, the cysts become inflammatory and spread to the trunk and genitalia. Usually the nose and limbs are spared. Lesions tend to persist for decades, especially on the malar cheeks and behind the ears. Scarring may be severe. (5)
Chloracne differs from acne vulgaris in a number of ways. Initial lesions of chloracne are open comedones and there are very few pustules or nodular lesions as in acne vulgaris. Sebaceous glands tend to undergo atrophy in chloracne, whereas in acne vulgaris there is increased sebaceous gland activity. Further, meibomian glands tend to be involved in chloracne, but not acne vulgaris. Finally, chloracne can occur in any age group unlike acne vulgaris which favors adolescence. (1)
The diagnosis of chloracne cannot be made from the histological study of skin biopsies. Pathology similar to severe acne vulgaris is seen. (2)
Treatment of chloracne is usually unsatisfactory and consists of oral isotretinoin, topical retinoids, and oral antibiotics. If exposure ceases, lesions will gradually improve over many years or decades.
Outcome of the Patient with the Highest Recorded Blood Level of TCCD and a Novel Therapy
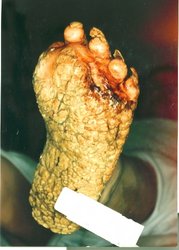
In 1997, a 30-year-old secretary had the highest recorded systemic levels of TCDD (144,000 units per gram of blood fat). (6) She was believed to have orally ingested TCDD at the chemical laboratory where she worked. During the first year after exposure, the patient had centrofacial inflammation and acne that gradually progressed to dense cysts covering the entire face with only a few lesions on the body. Over the next year, the patient's entire skin surface became covered with inflamed, painful cysts. The patient also developed palmoplantar keratoderma, which had not been previously reported as a manifestation of dioxin poisoning. In addition to skin disease, the patient suffered from chronic gastrointestinal pain with a resultant 10-kg weight loss and amenorrhea. The patient was treated unsuccessfully with a total cumulative dose of 13 g of isotretinoin (0.7 mg/kg body weight/day). She was then managed with oral antibiotics, methylprednisolone, and analgesics. The patient has required repeat surgical intervention for deep inflammation and cysts.
Interestingly, an exposed colleague of the patient who also had high levels of TCDD (26,000 units per gram of blood fat) experienced only mild symptoms with comedones and cysts limited to the malar crease which cleared with topical tretinoin.
A promising new approach to lowering blood TCDD levels was employed on both patients. Olestra, a non-absorbable, non-digestible fat substitute was given. The authors reported that TCDD levels decreased 8 to 10-fold using Olestra, reducing TCDD half life from about 7 to 1-2 years. (7)
Conclusion
Chloracne is the hallmark of dioxin toxicity and should alert the clinician to the diagnosis. The clinical severity of chloracne can vary significantly. Levels of TCDD can be measured in the blood to confirm the diagnosis. Therapy for chloracne is often unsatisfactory. Olestra is a promising new treatment for lowering systemic dioxin levels.
References
1. Dunagin WG. Cutaneous signs of systemic toxicity due to dioxins and related chemicals. J Am Acad Dermatol. 1984;10:688-700.
2. Health effects of Agent Orange and dioxin contaminants. JAMA. 1982;248(15):1895-7.
3. Mocarelli P, Garthoux PM, Brambilla P, et al. Dioxin health effects on humans twenty years after Seveso: a summary. In: Chemistry, Man, and Environment: the Seveso Accident 20 Years on Monitoring, Epidemiology, and Remediation. New York: Elsevier; 1999;41-51.
4. Carson K. Chloracne and other manifestations of dioxin toxicity. In: Demis DJ, ed. Clinical Dermatology. Philadelphia, Lippincott-Raven; 1995.
5. Tindal J. Chloracne and chloracnegens. J Am Acad Dermatol. 1985;13:539-558.
6. Geusau A, Abraham K, Geissier K, Sator M, Stingl G, Tschachler E. Severe 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) intoxication: clinical and laboratory effects. Environ Health Perspect. 2001;109:865-869.
7. Geusau A, Tschachler E, Meixner M, Sandermann S, Papke O, et al. Olestra increases faecal excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Lancet. 1999;354:1266-1269.
J. Barton Sterling MD, C. William Hanke, MD, MPH, FACP
Laser and Skin Surgery Center of Indiana
Address for Correspondence
C. William Hanke, MD, MPH, FACP
Laser and Skin Surgery Center of Indiana
13450 N. Meridian, Suite 355
Carmel, IN 46032
317-582-8484
E-mail: cwmhanke@sbcglobal.net
COPYRIGHT 2005 Journal of Drugs in Dermatology, Inc.
COPYRIGHT 2005 Gale Group