Abstract
In this report, a case is presented of a child, descendent from parents originating in the Virgin Islands, with symmetric and progressive hyperpigmented, hyperkeratotic plaques consistent with progressive symmetric erythrokeratoderma (PSEK). Additional family members were also affected in an autosomal dominant pattern of inheritance.
Erythrokeratodermas are rare genodermatoses that have characteristic clinical presentations of well-demarcated, hyperkeratotic, and erythematous plaques. Three types exist, differentiated by their clinical presentation. In this report, a case of progressive symmetric erythrokeratoderma (PSEK) is presented. The clinical features, pathogenesis, and treatment options for erythrokeratodermas are discussed.
Case Report
A healthy, 9-year-old African-American boy was seen at our center for lesions on the face and arms present since the age of two. Initially, the lesions presented as hyperpigmented patches and then progressed to plaques. The patient and mother denied any evanescent erythematous patches. The patient had some pruritus and had applied only emollients to the areas. There was a positive family history of similar findings in two brothers, one sister, the mother, and the maternal grandmother (Figure 1). The family is originally from the Virgin Islands.
[FIGURE 1 OMITTED]
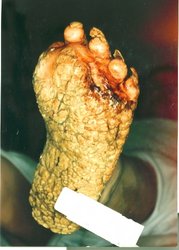
Physical examination revealed symmetric hyperpigmented and hyperkeratotic geometric plaques on the face, neck, axillae, and antecubital fossae (Figures 2a and 2b). There was no evidence of palmoplantar keratoderma. A biopsy was performed that showed papillated epidermal hyperplasia, hypergranulosis, compact orthokeratosis, and focal parakeratosis.
[FIGURE 2 OMITTED]
Discussion
The erythrokeratodermas are a group of rare genodermatoses that have similar clinical presentations as well-demarcated, hyperkeratotic and erythematous plaques, but differ in other clinical aspects and in their underlying genetic mutations. Three types exist based on their clinical presentation: erythrokeratoderma variabilis (EKV), erythrokeratoderma en cocardes (EKC), and progressive symmetric erythrokeratoderma (PSEK). All have a similar histopathologic picture that is nonspecific and includes acanthosis and papillomatosis with compact hyperkeratosis, basketweave hyperkeratosis, and parakeratosis.
EKV was first described in 1907 by DeBuy Wenniger and later named by Mendes da Costa in 1925 after describing the condition in a mother and daughter. With EKV, migrating erythematous patches usually present by age one and can last hours to days. In addition, patients have fixed hyperkeratotic plaques primarily on extensor surfaces, buttock and face (1,2). The mutation in EKV involves the GJB3 gene that encodes the gap junction protein connexin 311.
EKC was first described clinically in 1947 by Degos. It presents as hyperkeratotic plaques that appear early in infancy. Later, intermittent lesions appear that have concentric erythema and central scale (1). Although no specific gene mutations have been described for EKC, one case report in the literature has shown decreased expression of loricrin and keritin (1) in affected skin (3).
PSEK is a third variant, first reported by Darier in 1886, and formally presented in 1922 by Gottron. Although some sporadic cases have been noted, it is predominantly an autosomal dominant condition with incomplete penetrancel. It presents with symmetric, hyperkeratotic plaques distributed on the extremities, buttocks, and face (1,2). A palmoplantar keratoderma is seen in 50% of cases and is similar to that seen in the icthyotic variant of Vohwinkel syndrome. In these cases, also called loricrin keratodermas, frameshift mutations in the loricrin gene, a major structural component of the cornified cell envelope, have been identified (4). These mutations in loricrin appear to affect its nuclear and nucleolar function but not its role in cornified cell envelope crosslinking (5).
Treatment options include topical retinoids, emollients, and/or lactic acid. While oral retinoids have been shown to result in prolonged improvement, the side effect profile limits their long-term use, especially in young children (6). Of note, there have been reports of symptom regression in some patients with erythrokeratoderma (7).
References:
(1.) Textbook of Pediatric Dermatology, Vol II. Eds. Harper J, Oranje A, Prose N. Blackwell Science, Ltd., Osney Mead, Oxford, England: 1148-1152.
(2.) Macfarlane AW, Chapman SJ, Verbov JL. Is erythrokeratoderma one disorder? A clinical and ultrastructural study of two siblings. Br J Dermatol 1991; 124:487-491.
(3.) Ishida-Yamamoto A, Kelsell D, Common J, Houseman MJ, Hashimoto M, Shibaki H, et al. A case of erythrokeratoderma variabilis without mutations in connexin 31. Br J Dermatol 2000 Dec; 143(6):1283-7.
(4.) Ishida-Yamamoto A, McGrath JA, Lam H, Lizuka H, Friedman RA, Christiano AM. The molecular pathology of progressive symmetric erythrokeratoderma: a frameshift mutation in the loricrin gene and perturbations in the cornified cell envelope. Am J Hm Genet 1997; 61:581-589.
(5.) Ishida-Yamamoto A, Kato H, Kiyama H, Armstrong DKB, Munro CS, Eady RA, Nakamura S, Kinouchi M, Takahashi H, Iizuka H. Mutant loricrin is not crosslinked into the cornified cell envelope but is translocated into the nucleus in loricrin keratoderma. J Invest Dermatol 2000; 115:1088-1094.
(6.) Van de Kerkof PCM, Steijlen PM, Van Dooren-Greebe RJ, Happle R. Acitretin in the treatment of erythrokeratoderma variabilis. Dermatologica 1990; 181:330-333.
(7.) Salamon, T. Regression and disappearance of clinical symptoms in some cases of genodermatoses. Dermatology 1993; 186:245-247.
ADDRESS FOR CORRESPONDENCE:
Martha P Arroyo, MD PhD
The Ronald O. Perelman
Department of Dermatology
560 First Avenue
New York, NY 10016
Email: mp.arroyo@stanfordalumni.org
COPYRIGHT 2002 Journal of Drugs in Dermatology
COPYRIGHT 2003 Gale Group