Question: We have many surgical patients who come to our facility with untreated head lice (Pediculus humanus capitis), and we cancel the elective surgery until the patient is treated. Is this correct procedure? What controls should be implemented when the surgery is on emergency and cannot be delayed for treatment of the lice? How do we protect employees and patients from the transmission of lice?
Answer: Treating the patient before elective surgery may be the preferred practice. Literature shows that nosocomial transmission of head lice is rare but possible. Head lice are not vectors of specific serious disease, but if they are not treated, complications may arise, including secondary bacterial infections such as impetigo, pyoderma, and lymphadenopathy.(1)
Head lice infestations have been part of human history since the beginning of recorded time. Nit combs have been found in the Judean desert dating back to 68 AD. With the development of dichlorodiphenyltrichloroethane (ie, DDT), head lice was almost eradicated during World War II. Them was, however, a resurgence of head lice in the 1960s, and the incidence continues to rise.
No area in the United States is immune to head lice. An estimated six to 12 million people are affected each year. Children between the ages of five and 12, girls more often than boys, are affected most often. More infestations occur among children with brown or red hair than among those with black or blond hair. Some children are prone to repeated infestations, and others are unaffected. Large families are infested more often than small ones, probably due to proximity rather than socioeconomic status.(2)
Head lice are attracted to people with fine hair rather than coarse hair. Except for the African-American population, lice infestation occurs in all races and socioeconomic levels. A low incidence among African-American families is attributed to the shape of the hair shaft.(3) Individuals with good personal hygiene practices are more vulnerable than people with long, dirty hair and scalps. Head lice actually prefer a clean environment.(4)
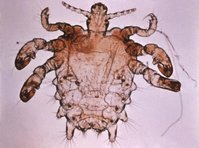
The head louse is an external parasite of the human host. It is 2 mm to 4 mm long with six clawlike legs and a flat, grayish-brown, wingless body. It lives on hair near the scalp where it finds food and warmth, feeding by sucking the host's blood. Itching is caused by injection of louse saliva into the scalp.
Transmission is through person-to-person contact by direct, even brief, head-to-head contact. Lice cannot jump or fly, but they can crawl up to 12 inches. During the average 30-day life span of the louse, the female attaches 60 to 150 eggs, commonly called nits, to hair shafts near the scalp with a sticky, gluey substance. The nits hatch into a nymph, or immature louse, in seven to 10 days. The louse reaches maturity in approximately two weeks.
The adult louse usually feeds every four to six hours, but it is capable of surviving for two days without a host. Nits will survive without a human host for up to 10 clays but will not hatch in temperatures below 71.6 [degrees] F (22 [degrees] C). Temperatures greater than 125 [degrees] F (52 [degrees] C) for a period of five minutes is lethal to nits and the adult louse.(6)
Many experts believe that transmission can occur indirectly by contact with clothing, bedding, or personal grooming items such as combs, hats, and scarves. In general, the risk of nosocomial head lice transmission is very low unless there is an opportunity for direct contact. Some authorities consider any head lice that fall off the scalp on pillows, clothing, or bed linens to be generally sick and unlikely to be able to establish themselves on another head.(7)
When delay of surgery is not a reasonable option, careful use of contact precautions and implementation of the AORN "Recommended practices for environmental cleaning in the surgical practice setting" should effectively minimize the risk of transmission.(8) The life cycle of the louse is dependent on availability of a human blood meal and moderate temperature. Louse viability is possible between 59 [degrees] F (15 [degrees] C) and 100.4 [degrees] F (38 [degrees] C), which are common temperatures found in most OR suites. Combs and brushes, if used, should be cleaned in hot water. All bed linens, pillows, towels, clothing, and nondisposable headwear should be placed in a sealed plastic laundry bag and laundered by the facility-approved commercial laundry. If a commercial laundry is not available, these items should be machine washed in hot water and dried in a hot dryer. Disposable supplies should be discarded in the normal appropriate containers and sealed. The OR should be cleaned according to routine procedures, using the hospital-approved disinfectant. Pesticide sprays are not necessary.(9)
Perioperative nurses and other health care workers exposed to patients with head lice do not require treatment unless they show evidence of infestation, t" Treatment for the patient or the unfortunate infested employee includes use of one of three currently available pesticidal agents: 1% lindane shampoo, 0.3% pyrethrin shampoo, or 1% permethrin cream rinse. The medication of choice is 1% permethrin cream rinse, which has been found to have the greatest efficacy and widest margin of safety. It is photostable and 99% ovicidal and does not require repeated applications.
After chemical treatment, the nits must be removed from the hair to avoid reinfestation. As the nit is glued to the hair and can be difficult to remove, a solution of 50% vinegar and 50% water can be used to loosen the nit's hold and ease removal. There also are commercially prepared products available to break the gluey bond. Follow-up checks, education, and examination of family members should be performed to prevent repeated infestation. Personnel with head lice should not provide patient care until after they receive treatment and are free from infestation on follow-up examination.(11)
Finally, be aware that the head louse is only one of three lice species found in humans. The other two species require slightly different approaches in patient care. The body louse, Pediculus humanus corporis, is a vector of epidemic typhus, trench fever, and relapsing fever. This parasite is associated with poverty and poor hygiene, and infestations are common among homeless and lower income people. This louse is sometimes called the "clothing louse" because it lives in the seams of clothing and leaves the clothing to feed on skin. It survives because the victim usually wears only one set of clothing. Transmission is conducted by contact with infested clothing or bedding.(12)
The third species is the pubic louse, Phthirus pubis, commonly known as the crab louse. This species also is nondiscriminatory. It will infest anyone of any race or socioeconomic status, and infestation is most common in sexually active adults. Transmission is by direct venereal skin-to-skin contact, and 95% of sexual contacts of an active carrier will develop an infestation. It is uncertain whether transmission of this type of louse occurs from bedding or clothing. Nosocomial transmission is considered to be very unlikely.(13)
Question: At the hospital where I previously worked, the use of formaldehyde was changed because of concerns with Occupational Safety and Health Administration (OSHA) safety requirements and ventilation issues. At the hospital where I am now employed, the nurses keep specimen containers in the ORs that are already filled with formalin. I would like to put a stop to this unsafe practice, but I need justification. Is there a specific OSHA standard or AORN recommendation that addresses the use and storage of formalin?
Answer: The safest approach is to store the formalin, which is 37% formaldehyde mixed in water, in a separate, well-ventilated, centralized location. Occupational exposure to formalin is regulated by OSHA standard 29 CFR 1910.1048. Formalin is a potential human carcinogen.
Exposure to airborne concentrations above 0.1 parts per million (ppm) can cause irritation of the eyes and upper airway. A 30-minute exposure to 100 ppm can be fatal, and pulmonary edema has been diagnosed after exposures of 50 ppm. These levels can be generated by small formaldehyde spills of only one pint or less, even in ventilated areas.(15) Blindness can result from splashes in the eyes, and dermal contact causes various degrees of reactions, including sensitization.(16) For these reasons, formalin should be stored and dispensed carefully in a centralized location. Individual specimen containers can be filled under controlled conditions and stored at the central location, close to eye wash, cleanup, and decontamination facilities in the event of an accidental spill or injury.
The OSHA standard requires that employers
* provide protective clothing and equipment, including goggles, face shields, and gloves;
* provide emergency showers;
* provide eye wash facilities in the immediate work area (bottle-type eye washes are not acceptable);
* label all containers of formalin; and
* provide employee education and training.
A spill or other accident involving formalin in the OR would be difficult to contain. The risk of hazardous exposure of formalin to patients and health care personnel should be minimized in any way possible. In many facilities, the surgical specimen is taken in an empty container directly from the OR to a centrally located room where the prefilled specimen containers are kept. The specimen is placed in the container in the room where formalin can be used and safely stored and the risks of hazardous spills are minimized. For additional information on the OSHA standard, contact OSHA at (202) 219-8036 or visit the OSHA web site at www.osha.gov.
Question: We have several staff members and physicians who chew gum in the OR and during procedures. I do not think that this is a good practice, but I am not sure why. Is this acceptable? Does AORN have a recommendation regarding this practice?
Answer: AORN does not have an official recommended practice that addresses this issue; however, we believe the practice should be avoided. The movement of the jaw beneath the surgical mask causes friction between the skin and the mask. This friction can cause shedding of the epidermis, known as scuff. The scuff can become airborne, be transported to any area of the surgical field, and can fall into the open wound.
The surgical mask serves to collect bacteria from the nasopharyngeal airway of the wearer. With the chewing motion and excessive talking, exhaled droplets containing bacteria are more likely to be expelled into the environment.(17)
Question: We are in the process of building an ambulatory surgery center, and the architect has proposed puffing scrub sinks inside the OR. I have never heard of this, and I think it would be a problem from an infection control standpoint. People involved with the change also have proposed placing a door to the outside from the restricted OR corridor to exit patients in an emergency. I have serious concerns about this. They are planning to have the changing rooms near the admitting area with an exit into the admitting corridor and one exit into the sterile hall. They really do not want nurses' input, but they clearly need help! What is AORN's position?
Answer: You are absolutely correct that this would be a serious breach of basic infection control practices. Scrub sinks should never be located in the OR and should be arranged in an area to minimize incidental splatter on nearby personnel, medical equipment, or supplies. Scrub sinks should be recessed into an alcove out of the main traffic areas. The alcove must be located off the semirestricted or restricted areas of the surgical suite. Scrub sinks must be located outside the sterile core.(18)
The exit from the OR corridor may be a fire safety building code issue. You would need to verify this with your local building department. This is not uncommon, but when the fire exit door is required, the door must be clearly marked for exit only and should not allow entry into the area from the outside.
Changing rooms are considered a "transition" area, and the proposed location may be acceptable, depending on how traffic is going to be controlled. Refer to AORN's "Recommended practices for traffic patterns in the perioperative practice setting."(19) Share this information with the architect and other members of the construction team to assist them in designing the facility for safe patient care.
For a full reference on construction guidelines, refer to Guidelines for Design and Construction of Hospital and Health Care Facilities, 1996-97.(20) If your facility library does not have a copy, you can order one by calling (800) 365-2724.
NOTES
(1.) Understanding Head Lice, Pediculus capitis; Infestation, and Management, A Guide for Healthcare Professionals (Care Technologies, Inc, 1996).
(2.) F Sokoloff, "Identification and management et pediculosis," Nurse Practitioner 19 (August 1994) 62-64.
(3.) Ibid.
(4.) S J McKay, "Myths and facts about head lice," Nursing99 29 (June 1999) 30.
(5.) L A Lettau, "Nosocomial transmission and infection control aspects of parasitic and ectoparasitic diseases part III. Ectoparasites/summary and conclusions," Infection Control and Hospital Epidemiology 12 (March 1991) 179-185.
(6.) Sokoloff, "Identification and management of pediculosis," 62.
(7.) Lettau, "Nosocomial transmission and infection control aspects of parasitic and ectoparasitic diseases part III. Ectoparasites/summary and conclusions," 179.
(8.) Ibid; "Recommended practices tar environmental cleaning in the surgical practice setting," Standards, Recommended Practices, and Guidelines (Denver: Association of Operating Room Nurses, Inc, 1999) 233-238.
(9.) Sokoloff, "Identification and management of pedicuiosis," 63.
(10.) R Sharbaugh, "The risk of occupational exposure and infection with infectious disease," Nursing Clinics of North America 34 (June 1999) 493-508.
(11.) E A Bolyard et al, "Guideline for infection control in healthcare personnel, 1998, Hospital Infection Control Practices Advisory Committee," Infection Control and Hospital Epidemiology 19 (June 1998) 407-463.
(12.) Lettau, "Nosocomial transmission and infection control aspects of parasitic and ectoparasitic diseases part III. Ectoparasites/summary and conclusions," 179-180.
(13.) Ibid, 180.
(14.) OSHA Directives, CPL 2-2.52, "Enforcement Procedure for Occupational Exposure to Formaldehyde." Available from http://www.osha-slc.gov/OshDoc/ Directive_data/CPL_2-2_52.html. Accessed 20 Dec 1999.
(15.) Ibid.
(16.) OSHA Fact Sheets: 01/01/1995. "Occupational Exposure to Formaldehyde." Available from http://www. osha-sic.gov/OshDoc/Fact_data/FSNO95-27.html. Accessed 20 Dec 1999.
(17.) D Fogy, "OR attire; endoscopic equipment; artificial nails and chewing gum in the OR; monitoring patients," AORN Journal 54 (August 1991) 335-339.
(18.) The American Institute of Architects Academy of Architecture for Health with assistance from the US Department of Health and Human Services, Guidelines for Design and Construction (Hospital and Health Care Facilities, 1996-97 (Washington, DC: The American Institute of Architects Press, 1996) 24.
(19.) "Recommended practices for traffic patterns in the perioperative practice setting," in Standards, Recommended Practices, and Guidelines (Denver: AORN, Inc, 2000) 365-367.
(20.) The American Institute of Architects Academy of Architecture for Health with assistance from the US Department of Health and Human Services, Guidelines for Design and Construction of Hospital and Health Care Facilities, 1996-97, 24.
COPYRIGHT 2000 Association of Operating Room Nurses, Inc.
COPYRIGHT 2001 Gale Group