The immediate puerperium, the time between delivery and hospital discharge, represents an important opportunity for the physician to review contraceptive options with the patient. The need for effective contraception in the postpartum period is well recognized, since women who choose not to breast-feed could resume having ovulatory cycles before the traditional 6 or 8 weeks postpartum visit. [1] Women who are given bromocriptine for the suppression of physiologic lactation could ovulate as soon as 2 to 3 weeks after deliver. [2]
Even though there is the potential for the postpartum woman to become pregnant again, the inherent risks of contraceptive methods must be evaluated critically during this period. Specifically, oral contraceptives can present an increased risk of thromboembolism in the immediate postpartum period. The following is a case presentation and review of the issues concerning thromboembolism associated with the use of oral contraceptives in the early postpartum period.
Case Report
The patient was a 21-year-old woman (gravida 2, para 2) who was 4 weeks postpartum when she first noted the onset of pain and swelling in her right lower calf and a mottled discoloration from the proximal thigh to her toes. The pain worsened with walking and radiated to her upper thigh. She denied any trauma to the area and did not have a history of phlebitis or pulmonary embolism. Her only medication was a triphasic oral contraceptive (Triphasil), which had been started 1 week after delivery. The patient reported that she had just finished her first pack of pills. Her social history was significant in that she smoked two to three cigarettes daily. Her height was 165 cm (5 ft 5 in.) and she weighed 74.25 kg (165 lb).
Her medicl history was significant only for her most recent pregnancy, which had been complicated by early vaginal bleeding, a relatively sedentary lifestyle, and a weight gain of 18 kg (40 lb). She had had a spontaneous vaginal delivery complicated by a hemorrhage in the third stage of labor. The hemorrhage was controlled with uterine message, intravenous oxytocin, and oral methylergonovine maleate.
The patient was admitted to the hospital with the diagnosis of a probable deep venous thrombosis (DVT) for which she received an intravenous bolus of 5000 U of heparin followed by 1000 U per hour intravenously. An impedance plethysmograph subsequently documented a DVT in her right leg. An electrocardiogram showed inverted T waves in leads [V.sub.1] through [V.sub.3], so a ventilation-perfusion (V/O) scan was ordered. On her way to the nuclear medicine department to have the test, the patient began to experience pleuritic chest pain on the left side and diaphoresis. A pulmonary embolism was subsequently documented on the V/Q scan (Figures 1 and 2). The patient received heparin and warfarin during the hospitalization. She was discharged 13 days after admission, given a prescription for warfarin, 7.5 mg daily, and advised to use thromboembolic disease stockings. The patient and her husband chose to use barrier contraceptives until a tubal ligation could be performed.
Discussion
During pregnancy, the elements of Virchow's triad, including stasis, vascular damage, and hyperoagulability, may all be present. [3] Hypercoagulability may result from changes in both the coagulation cascade and the fribrinolytic system. Circulating estrogens induce the hepatic production of procoagulant factors VII, VIII, IX, and X. Increases in the concentrations of prothrombin and fibrinogen may also occur. [4,5] The concentrations of protein C and protein S, inhibitors of the coagulation cascade, may also be reduced. [4]
In the postpartum period, a slow decline in the concentration of estrogen-induced clotting factors is accompanied by the return of normal activity within the fibrinolytic system in the first few days following delivery of the placenta. For some women, however, the changes in the procoagulant factors may persist for at least 7 to 10 days postpartum. [6] During pregnancy, the incidence of thromboembolic disease has been estimated to be as high as 0.15% for superficial thrombophlebitis and 0.36% for deep venous thrombophlebitis. In the postpartum period, because of the thromotic changes described as well as the stasis and the vascular damage following a delivery, the incidence may be substantially greater than that during pregnancy. [3]
Since the introduction of oral contraceptives almost 30 years ago, epidemiologic data on the association between their use and the development of venous thromboembolism have been well documented. [7] The use of oral contraceptives containing less than 100 [microgram] of either ethinyl estradiol or mestranol has approximately one third the risk of thromboembolism that using those products containing 100 [microgram] or more of the estrogen component has. [8] Whether oral contraceptives containing 35 [microgram] or less of the estrogen component definitely are safer, with respect to thromboembolism, than the 50 [microgram] and 80 [microgram] products is unknown because data are conflicting. [8,9] In addition to the dose of the estrogen, progestins such as norethindrone and ethynodiol diacetate have inherent estrogenic activity. It is unknown if triphasic oral contraceptives offer advantages in terms of reduced risk of venous thromboembolism.
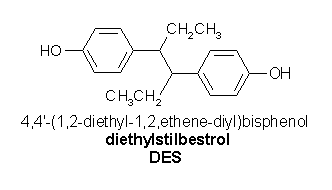
For the postpartum woman, basic concerns about the relative risk of thromboembolism with oral contraceptives become even more important. Information on the actual effect of oral contraceptives in placing a postpartum woman further at risk are unavailable; data are derived primarily from studies using high-dose estrogens, especially stilbestrol, to suppress lactation. [6,10] Von Kaulla et al [6] demonstrated that the use of high-dose stilbestrol in the immediate postpartum period delays the reversal of the hypercoagulable state induced by pregnancy by at least 2 to 3 weeks.
In the present case, the patient clearly had other risk factors for thromboembolism in addition to the administration of an oral contraceptive in the postpartum period. For example, smoking produces an additive effect to the risk of using oral contraceptives. Of the risk factors studied (obesity, immobilization, increasing age, blood group type, surgery, chronic disease, etc), smoking remains the single most important factor in combination with an oral contraceptive; heavy smoking can increase the risk of thromboembolism 23-fold. [11] In this particular young woman, the use of the oral contraceptive in the immediate postpartum period may have been the final factor that precipitated her thromboembolic event.
Standard references including Williams Obstetrics [1] and the Physicians' Desk Reference [12] do not provide a consensus regarding the optimal time for restarting oral contraceptives in the postpartum period. In Williams Obstetrics it is suggested that oral contraceptives be restarted during the third week postpartum. The recommendations from the manufacturers, however, range from stating that oral contraceptives may be started in the immediate postpartum period to indicating that the products should not be prescribed earlier than a minimum of 4 to 6 weeks postpartum.
In using any contraceptive in the postprtum period, the goal should be early and effective contraception. Counseling may include a discussion of the potential problems of having to care for two children under the age of 12 months, as well as of the risks and benefits of the available methods of contraception acceptable for use in the postpartum period. If an oral contraceptive is chosen, it should be initiated only after at least 2 weeks postpartum and should include on the prescription label the actual date to begin taking the pills. If significant risk factors for thromboembolism are present, the physician should delay initiating oral contraceptives for at least 4 to 6 weeks postpartum. Written instructions should be provided on what contraception is to be used in the interim and what to do if menses return before the postpartum visit. To reinforce compliance, the physician should discuss any questions or concerns with the couple at their newborn's first visit 1 to 2 weeks postpartum.
Progestin-only contraceptives include norethindrone, norgestrel, and medroxyprogesterone acetate. While these have been suggested as possible alternatives in the postpartum period, the potential risk of thromboembolism remains. [12]
Conclusions
The current low-dose monophasic and triphasic oral contraceptives have been used safety and effectively by millions of women. Their use in the postpartum period, however, may present specific risks; their contraceptive efficacy must be balanced with their potential to increase the risk of thromboembolism. Oral contraceptives should be initiated only after 2 weeks or more postpartum in women without other risk factors for thromboembolism, and only after 4 to 6 weeks or more postpartum in other women.
References
[1] Cunningham FG, MacDonald PC, Gant NF. Williams Obstetrics. 18th ed. Norwalk, Conn: Appleton & Lange, 1989.
[2] Kremer JA, Rolland R, Van Der Heijden PF, et al. Return of gonadotropic function in postpartum women during bromocriptine treatment. Fertil Steril 1989; 51:622-7.
[3] Weiner CP. Diagnosis and management of thromboembolic disease during pregnancy. Clin Obstet Gynecol 1985; 28:107-18.
[4] Finley BE. Acute coagulopathy in pregnancy. Med Clin North Am 1989; 73:723-43.
[5] Pechter L, Alexander B. Increased clotting factors in pregnancy. N Engl J Med 1961; 265:1093-6.
[6] Von Kaulla E, Droegemueller W, Aoki N, von Kaulla KN. Effect of estrogens on postpartum hypercoagulability and antithrombin III activity. Am J Obster Gynecol 1972; 113:920-6.
[7] Sartwell PE, Masi AT, Arthes FG, et al. Thromboembolism and oral contraceptives: an epidemiologic case-control study. Am J Epidemiol 1969; 90:365-80.
[8] Helmrich SP, Rosenberg L, Kaufman DW, et al. Venous thromboembolism in relation to oral contraceptive use. Obstet Gynecol 1987; 69:91-5.
[9] Meade TW, Greenberg G, Thompson SG. Progestogens and cardiovascular reactions associated with oral contraceptives and a comparison of the safety of 50 microgram and 30 microgram estrogen preparations. Br Med J 1980; 280:1157-9.
[10] WHO Task Force on Oral Contraceptives. contraception during the postpartum period and during lactation: the effects on women's health. Int J Gynaecol Obstet 1987; 25 (suppl):13-26.
[11] Hafez, ESE, ed. Human reproduction: conception and contraception. 2nd ed. New York: Harper and Row, 1980:595.
[12] Physicians' Desk Reference. 44th ed. Oradell, NJ: Medical Economics Co, Inc, 1990.
COPYRIGHT 1991 Dowden Health Media, Inc.
COPYRIGHT 2004 Gale Group