METHOD OF PREPARATION
Note: It is generally recommended to prepare sufficient material for one or two additional suppositories due to loss during preparation. It may be necessary to calibrate the desired mold to be used prior to preparing this prescription.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Using an appropriate heat source, melt the required quantity of base for the number of suppositories to be prepared.
4. Pulverize sufficient sumatriptan tablets to produce the required amount of sumatriptan for the number of suppositories to be compounded.
5. Slowly sprinkle the powders into the melt with stirring until a uniform mixture is obtained.
5. Cool slightly, then pour into the mold in a continuous motion, if appropriate for the molds being used.
6. Cool, trim if necessary, package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed.
STABILITY
A beyond-use date of up to 6 months is appropriate for this preparation.1
USE
Sumatriptan suppositories have been used in the treatment of migraine headaches when an oral tablet or a nasal spray is not appropriate.
QUALITY CONTROL
Quality-control assessment can include weight, specific gravity, active drug assay, color, texture of surface, appearance, feel, dissolution test, physical observation and physical stability.2
DISCUSSION
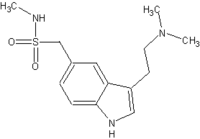
Sumatriptan succinate (C^sub 14^H^sub 21^N^sub 3^O^sub 2^S.C^sub 4^H^sub 6^O^sub 4^, MW 413.49) occurs as a white or almost-white powder that is freely soluble in water. A 1% solution in water has a pH of 4.5 to 5.3 and it should be protected from light. Fifty mg of sumatriptan is contained in approximately 70 mg of Sumatriptan succinate.3
Orally administered, sumatriptan is rapidly, but incompletely absorbed, and undergoes first-pass metabolism resulting in a low absolute bioavailability of only about 14%. Intranasally, bioavailability is 16% relative to subcutaneous administration. Sumatriptan has an elimination half life of about 2 hours. Rectal administration may increase the bioavailability of sumatriptan relative to oral administration.3
Polybase is a preblended water soluble-water miscible suppository base consisting of a homogenous mixture of various molecular weight polyethylene glycols. It contains polyethylene glycols (average molecular weight about 3440) and polysorbate 80. It is used in the preparation of suppositories and lozenges where a water soluble or water-miscible base is preferred. It releases its medication by dissolving in the local fluids. It is a white solid with a mild polyethylene glycol odor and has a specific gravity of 1.177. Products prepared from this base should not be stored or dispensed in polystyrene containers.
Polyethylene glycols (PEGs) can be blended in various ratios to arrive at suppository bases with differing characteristics that can accommodate certain quantities of liquids or solids.
Polyethylene glycol (Carbowax, PEG, polyoxyethylene glycol) is an addition polymer of ethylene oxide and water. At room temperature, polyethylene glycols with molecular weights of 200-600 are liquid and those with molecular weights greater than 1000 are solid. The liquid polyethylene glycols are clear, colorless or slightly yellow-colored, viscous liquids with a slight, but characteristic odor and a bitter, slightly burning taste. The density of the liquid PEGs is in the range of 1.11 to 1.14 g/mL. Solid polyethylene glycols are white or off-white pastes or waxy flakes. Those with molecular weights greater than 6000 are available as free-flowing powders. The density of the solid PEGs is in the range of 1.15-1.21 g/mL. The polyethylene glycols are all soluble in water and miscible in all ratios with other PEGs. The PEGs are chemically stable, do not support microbial growth and do not become rancid. When applied topically, especially to mucous membranes, they may cause irritation or stinging.4
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary 22. Rockville, MD: US Pharmacopeial Convention, Inc.; 2004: 2345-2349.
2. Allen Jr LV. Standard operating procedure for performing physical quality assessment of suppositories, troches, lollipops and sticks. IJPC 1999; 3(1): 56-57.
3. Sweetman SC, ed. MARTINDALE: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002: 456-458.
4. Price JC. Polyethylene glycol. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ad. Washington, DC: American Pharmaceutical Association; 2000: 392-398.
Copyright International Journal of Pharmaceutical Compounding May/Jun 2004
Provided by ProQuest Information and Learning Company. All rights Reserved