Abstract
The pharmacokinetics of an extemporaneous 25-mg suppository formulation of sumatriptan were compared to those of the marketed 25-mg oral tablet. Sixteen healthy volunteers enrolled in this open-label, two-way crossover study. Fifteen subjects completed the study. The pharmacokinetics of the suppository and the oral tablet were significantly different. T^sub max^ was observed at 0.5 hours in 12 of 15 subjects with the extemporaneous suppository, compared with the range of 0.75 hours to 1.5 hours in 13 of 15 subjects with the oral tablet. The mean C^sub max^ and area under the plasma concentration time curve were 5.4-fold and fourfold greater for the suppository than for the oral tablet. Both formulations were well tolerated, with mild headache experienced in only three subjects. Based upon its pharmacokinetic profile, the extemporaneous suppository may represent a useful alternative therapeutic administration route for some patients.
Introduction
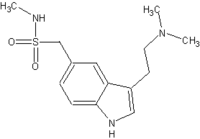
Sumatriptan was the first selective serotonin receptor agonist to be commercially available for the treatment of acute migraine headache.1 Its pharmacologic properties are well known and involve subpopulations of the serotonin (5-HT) receptors located in the cerebral and dural blood vessels. Although its precise mechanism remains unclear, sumatriptan has been suggested to produce vasoconstriction of the cranial vessels in the carotid circulation via the 5-HT1B and 5-HT1D receptors, resulting in pain relief.2,3 Sumatriptan is commercially available in an oral tablet, nasal spray and subcutaneous injection. A suppository formulation is available only in Europe.4 Self-administration of sumatriptan during an acute migraine headache has been shown to be practical and well accepted among patients, with demonstrated cost benefits in reducing healthcare expenditures.5-7
For patients who have migraine headache associated with nausea and vomiting, administration of the oral tablet is not feasible; other patients may prefer to avoid subcutaneous injections, and the nasal spray causes nasal irritation in some patients.4 For these patients, a sumatriptan suppository formulation can be a viable alternative to the marketed products. Results from a randomized clinical trial (n=184) showed that the 25-mg suppository was significantly more effective than the 12.5-mg suppository and placebo in reducing headache symptoms as early as 30 minutes after administration, with a sustained benefit for more than 2 hours.8
The pharmacokinetic disposition of sumatriptan in different formulations has been published only on a limited basis.8,9 When the 25-mg oral tablet was compared with the 25-mg suppository, the bioavailability of the suppository was significantly greater than that of the oral tablet in healthy volunteers.8 However, the formulation for the suppository's constituents was not provided. In another study, four different sumatriptan suppository doses were evaluated, from 12.5 mg to 100 mg, under single- and multiple-dose administration without an oral-tablet comparison in healthy volunteers.9 The suppository consisted of the drug base Witepsol W32, and the different doses exhibited a dose-dependent pharmacokinetic disposition. The purpose of this study was to compare the pharmacokinetic disposition in healthy volunteers of the single-dose 25-mg oral sumatriptan tablet with that of a 25-mg extemporaneous suppository using a Polybase formulation.
Methods
Mercer University's institutional review board approved the study, and we obtained informed consent from each subject. Sixteen nonsmoking healthy subjects (mean age, 24.9 ± 3.3 years; mean weight, 147.1 ± 24.5 lbs.; eight men and eight women) were selected. The volunteers had no history of cardiovascular, renal, hepatic, neurologic or gastrointestinal diseases. Each subject had a complete physical exam, ECG, blood tests (liver function, electrolytes, glucose, serum creatinine, blood urea nitrogen, complete blood count with differential) and urinalysis; and for the women in the study, the exam included a serum pregnancy test. All physical and laboratory results were within normal limits. All subjects were instructed to abstain from caffeine for 18 hours before drug administration. Subjects were not taking any concomitant medications before or during the study except for three women who were taking oral contraceptives (minimum time period of 6 months).
Sumatriptan, as the succinate salt (Imitrex, 25-mg tablets, Lot 9ZP0660) was provided by GlaxoSmithKline, Research Triangle Park, North Carolina. Prior to administration, each suppository was prepared individually at the Center for Clinical Research; and each suppository was used within 24 hours. Each extemporaneous suppository contained one 25-mg sumatriptan tablet (average weight, 0.151 g) crushed into a fine powder using a mortar and pestle. Polybase 2.34 g (Lot 8D6865, NDC 0574-0309-16, Paddock Laboratories, Inc., Minneapolis, Minnesota) was melted slowly in a water bath (at approximately 50°C). The amount of Polybase was calculated based upon the sumatriptan displacement factor of 0.92 and the average weight of the blank placebo Polybase suppository weight of 2.5 g. The method of determining the displacement factor was based upon a previous study using Polybase.10 The melted base was added to the powdered tablet mass. The mixture was stirred and poured into a suppository mold in a continuous layer. The excess base was trimmed after cooling for 30 minutes. Each suppository was wrapped individually in aluminum foil and stored in a plastic container and refrigerated until use.
The study was an open-label, two-way crossover design. Placebo comparisons were not used because of the strict pharmacokinetic design of the study. Each subject received the dosage formulation in the following order: oral tablet and then extemporaneous suppository. There was a washout period of 1 week between administration of each formulation.
Subjects fasted from midnight before drug administration to 4 hours afterward but could have unrestricted water intake. The oral sumatriptan tablet was ingested with 8 oz water. A glycerin suppository was administered 12 hours prior to the sumatriptan suppository to evacuate the bowel. This is a standard procedure to evaluate rectally administered medications. Subjects were instructed to insert the sumatriptan suppository with their gloved indexed finger to the depth of the distal interphalangeal joint and to retain the suppository for at least 4 hours.
The blood-sampling times varied depending upon the dosage-administration route. The times were based upon the elimination half-life of sumatriptan and the possible slower absorption rate of the suppository. Five milliliters of whole blood samples was obtained prior to administration and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10 and 12 hours after administration of the oral tablet. For the suppository, 5-mL blood samples were obtained before administration and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16 and 24 hours after administration. For both dosage formulations, blood samples were obtained via an indwelling venous catheter (Jelco 4056, Johnson & Johnson, Arlington, Texas) placed before drug administration. The catheter was flushed after each sample and every hour with 1 mL of heparin sodium 10 units/mL. The catheter was removed after the 8-hour sample was obtained, and the remaining samples were obtained by venipuncture. Blood samples were collected in a vacutainer tube containing heparin sodium. The samples were centrifuged within 30 minutes at 3,000 rpm for 30 minutes. The resulting plasma was immediately transferred to polypropylene tubes and stored at -80°C until the assay.
The plasma samples were assayed for sumatriptan by highperformance liquid chromatography (HPLC), with electrochemical detection by the method published by Andrews et al.11 Plasma (1.0 mL) was made basic with 0.1 mL of 4 M sodium hydroxide solution. A volume of 2.6 mL of methylene chloride: ethyl acetate (20:80) was added to extract the sumatriptan. The mixture was mixed on a vortex for 15 minutes and then centrifuged at 1,500 rpm for 4 minutes. A 2.0-mL portion of the upper layer was quantitatively transferred to a clean tube. Hexane (2 mL) and potassium phosphate buffer pH 7.0 (300 µL) were added. The mixture was mixed and centrifuged as described above. The tube was then immersed in a freezing mixture of acetone and solid carbon dioxide to freeze the lower aqueous layer. The upper layer was aspirated off. Following thawing, a 200-µL aliquot of the remaining aqueous solution was injected into the HPLC. The HPLC system consisted of a manual injector, a binary pump (Model G1312A, Flcwlctt Packard, Avondale, Pennsylvania), a column oven (Model 655A-52, Hitachi, Tarrytown, New York), and an electrochemical detector (Model 1049, Hewlett Packard). Data were processed with Hewlett Packard ChemStation software. The stainless-steel octadecyl silane column was 150 mm x 4.6 mm (Waters Spherisorb ODS-1 5 µm, Alltech Associates, Seerfield, Illinois). The column was equipped with a guard cartridge also packed with Spherisorb ODS-1. The mobile phase was composed of aqueous potassium phosphate buffer pH 7.0 and methanol (40:60). The flow rate was 1.0 mL/ minute, and the column was maintained at a temperature of 40°C. The electrochemical cell was set at +0.80 V. The lower limit of detection was 2.0 ng/mL. The standard curve was between 6 and 45 ng/mL (r^sup 2^ = 0.981), with an interassay and intra-assay coefficient variation of less than 10%. Each sample was run in duplicate.
The pharmacokinetics of sumatriptan were determined by model-independent methods with nonlinear least-squares regression analysis (WinNonlin Scientific Consulting, Inc., Gary, North Carolina). The area under the plasma concentration-time curve (AUC) from time zero to the last time point (12 hours for the oral tablet and 24 hours for the extemporaneous suppository) was calculated by the trapezoidal rule. The elimination half-life (t^sub 1/2^) was calculated as In 2/k^sub el^. Systemic clearance was determined by dividing the dose by the AUC. The maximum plasma concentration (C^sub max^) was the highest observed plasma concentration measured for each subject. The time to maximum plasma concentration (T^sub max^) was the time at which C^sub max^ occurred. All data were reported as mean ± standard deviation. Comparisons between the pharmacokinetic values for each formulation were made by a two-tailed Student's t-test. An a priori level of significance was set at 0.05.
Results
Fifteen of the sixteen subjects who were enrolled completed both treatment phases of the study. One subject developed influenza after completion of the oral-tablet phase and discontinued participation in the extemporaneous suppository phase. Pharmacokinetic data were available for 15 subjects. Pharmacokinetic differences in sumatriptan between men and women (data on file) were not found. A summary of the mean pharmacokinetic parameters of sumatriptan data for the oral tablet and the rectal suppository is presented in Table 1. A wide interpatient variability of plasma sumatriptan concentrations was observed. The sumatriptan plasma concentrations displayed in Fig. 1 from the extemporaneous suppository showed that higher drug concentrations are achieved at an earlier time point than with the oral tablet. Plasma sumatriptan concentrations were also higher at each time point with a corresponding significantly greater AUC than with the oral tablet. With the exception of T^sub max^, the remaining pharmacokinetic parameters for the extemporaneous suppository were significantly different than for the oral tablet. T^sub max^ was achieved at 0.5 hours in 12 of the 15 subjects following sumatriptan suppository, while T^sub max^ was delayed to 4.0 hours for the remaining 3 subjects. The T^sub max^ ranged from 0.75 hours to 1.5 hours following the oral tablet in 13 of the 15 subjects. The two remaining subjects' T^sub max^ occurred at 2.5 hours and 3.0 hours, respectively.
The mean AUC and C^sub max^ of the suppository were about fourfold and 5.4-fold greater than those of the oral tablet, respectively. Subsequently, the mean elimination half-life for the suppository was 2.6-fold longer than for the oral tablet; and the mean clearance was 3.5-fold lower than for the oral tablet. Both formulations of sumatriptan were safe and well tolerated by all subjects. Mild headache was reported in only three subjects (one = oral tablet, two = rectal suppository). Only one subject had received a single dose of acetaminophen 650 mg for his/her headache, which was effective in relieving the pain.
Discussion
Results regarding disposition of the sumatriptan oral tablet observed in this study (Table 1) are similar to those previously reported in pharmacokinetic studies.1 A double absorption peak observed in our study (Figure 1) also resembles previously reported clinical data.8,11 This study further provides data on an extemporaneous suppository formulation with Polybase used in healthy men and women volunteers. These results indicate that the pharmacokinetic disposition of the sumatriptan extemporaneous suppository used in this study significantly differs from that of the oral tablet, and the results suggest that the amount of sumatriptan absorbed from the suppository route is considerably greater.
Data regarding the pharmacokinetic disposition of sumatriptan from an extemporaneously compounded suppository are limited. This study provides data on an extemporaneous suppository formulation with Polybase used in healthy men and women volunteers. These results indicate that the pharmacokinetic disposition of sumatriptan from the extemporaneous suppository significantly differs from that of the oral tablet and suggests that the amount of sumatriptan absorbed from the suppository is considerably greater.
Our results indicate greater systemic availability of sumatriptan from the suppository formulation, which is consistent with data from Duquesnoy et al,8 where the relative bioavailability of the suppository was 134% greater than that of the oral tablet. Interestingly, their results also showed that the AUC values for the suppository were greater than those for the other routes of administration, including both nasal and subcutaneous injection.
The increased amount of sumatriptan from the suppository formulation can be explained by various factors. A previous study reported that less than 5% of a sumatriptan dose and about 18% of its indole acetic acid metabolite were renally excreted (Unpublished data, GlaxoSmithKline). From the oral tablet, the renal excretion for sumatriptan and its indole metabolite is about 2% and 35%, respectively.8 According to the urinary excretion data, the relative bioavailability of the suppository should be at least twice that of the oral route. Therefore, a smaller fraction of sumatriptan could be metabolized by rectal administration compared with the oral route, resulting in greater drug concentrations in the body.9
Sumatriptan was rapidly absorbed from the suppository in 80% of the subjects, as the concentrations were easily detectable at the 0.5-hour time period. This indicates that the drug is readily absorbed through the rectal membranes. Although three subjects had a longer T^sub max^ of 4.0 hours, the AUCs for these subjects ranged between 179.0 ng * hours/mL to 269.4 ng * hours/mL, which indicates sumatriptan exposure comparable to that for the other subjects. The delay in the T^sub max^ for these subjects may be due to the placement of the suppository during insertion. Absorption from the rectal route was greater than from the oral in this study, which is also similar to the results of Duquesnoy et al,8 who reported that the median T^sub max^ of 1.0 hours for the suppository was earlier than the median time of 1.5 hours for the oral tablet. As previously mentioned, the suppository formulation was not presented in the study by Duquesnoy et al.8 The use of Polybase in this study appears to yield a desirable pharmacokinetic profile for rectal sumatriptan administration that includes a rapid absorption and greater relative bioavailability than the oral tablet.
Another interesting finding in this study is that the mean maximal plasma drug concentrations were higher and the mean elimination half-life was longer than in previously reported studies.1,12 An explanation for these occurrences may be partially explained by the suppository formulation, which may have resulted in increased absorption from the rectal membranes. In addition, rectal administration of the drug likely reduced its first-pass drug metabolism, resulting in reduced systemic clearance. Despite the higher C^sub max^ and AUC with the suppository, sumatriptan was well tolerated in this study, and adverse events were similar to those observed by sumatriptan when administered by other routes.1,2,12,13 The longer mean elimination half-life with the extemporaneous suppository could be a desirable factor in prolonging therapeutic benefit during an acute migraine headache in patients.
In conclusion, the pharmacokinetic profile of an extemporaneous sumatriptan Polybase suppository formulation has been compared with that of an oral tablet. The results from this study suggest that a patient with an acute migraine headache that requires a 50-mg or 100-mg sumatriptan tablet would benefit from a lower drug dose administered via the rectal route, such as the 25-mg extemporaneous suppository made with Polybase. Although clinical studies would be needed to confirm this suggestion, the extemporaneous suppository, based upon its disposition, may represent a useful therapeutic approach as an alternative route of administration for the treatment of migraine headache.
References
1. Perry CM, Markham A. Sumatriptan. An updated review of its use in migraine. Drugs 1998;55:889-922.
2. Razzaque Z, Heald MA, Pickard JD et al. Vasoconstriction in human isolated middle meningeal arteries: Determining the contribution of 5-HT1B and 5-HT1F-receptor activation. Br J Clin Pharmacol 1999;47:75-82.
3. Nilsson T, Longmore J, Shaw D et al. Contractile 5-HT1B receptors in human cerebral arteries: Pharmacological characterization and localization with immunocytochemistry. Br J Pharmacol 1999;128:1133-1140.
4. Tepper SJ, Cochran A, Hobbs S et al. Sumatriptan suppositories for the acute treatment of migraine. S2B351 Study Group. Int J Clin Pract 1998;52:31-35.
5. Gobel H, Baar H, Beikufner HD et al. Practicability and acceptance of subcutaneous self-administration of the selective serotonin agonist Sumatriptan. Headache 1998;38:267-269.
6. Biddle AK, Shih YC, Kwong WJ. Cost-benefit analysis of Sumatriptan tablets versus usual therapy for treatment of migraine. Pharmacotherapy2000;20:1356-1364.
7. Joish VN, Cady PS. Effect of Sumatriptan on health care resource use among patients with migraine. Manag Care Interface 2001;14:68-72.
8. Duquesnoy C, Mamet JP, Sumner D etal. Comparative clinical pharmacokinetics of single doses of Sumatriptan following subcutaneous, oral, rectal and intra nasal administration. Eur J Pharm Sci 1998;6:99-104.
9. Kunka RL, Hussey EK, Shaw S et al. Safety tolerability, and pharmacokinetics of Sumatriptan suppositories following single and multiple doses in healthy volunteers. Cephalalgia 1997;17:532-540.
10. Fox JL, Ward ES, Tenjarla S. Formulation, dissolution and efficacy of an extemporaneously prepared Zofran suppository dosage formulation. Presented at: The 142nd Annual Meeting and Exposition of the American Pharmaceutical Association; March 18-22,1993; Orlando, FL.
11. Andrew PD, Birch HL, Phillpot DA. Determination of sumatriptan succinate in plasma and urine by high-performance liquid chromatography with electrochemical detection. J Pharm Sci 1993;82:73-76.
12. Tfelt-Hansen P. Efficacy and adverse events of subcutaneous, oral, and intranasal sumatriptan used for migraine treatment: A systematic review based on number needed to treat. Cephalalgia 1998;18:532-538.
13. Imitrex [package insert]. Research Triangle Park, NC:GlaxoSmithKline.
Hiral D. Desai, PharmD
Kara L. Shirley, PharmD
Scott R. Penzak, PharmD
J. Grady Strom, PhD
Yuen Yi Hon, PharmD
Vicky Spratlin, MD
Michael W. Jann, PharmD
Mercer University Southern School of Pharmacy
Atlanta, Georgia
This study was supported by a grant-in-aid from GlaxoSmithKline, Research Triangle Park, North Carolina.
Address correspondence to: Michael W. Jann, PharmD, Department of Clinical and Administrative Sciences, Mercer University, Southern School of Pharmacy, 3001 Mercer University Dr., Atlanta, GA 30341.
E-mail: jann_mw@mercer.edu
Copyright International Journal of Pharmaceutical Compounding Nov/Dec 2003
Provided by ProQuest Information and Learning Company. All rights Reserved