Definition
Genetic testing examines the genetic information contained inside a person's cells to determine if that person has or will develop a certain disease or could pass a disease to his or her offspring.
Purpose
Some families or ethnic groups have a higher incidence of a certain disease than does the population as a whole. Before having a child, a couple from such a family or ethnic group may want to know if their child would be at risk of having that disease.
Early in pregnancy, the baby's cells can be studied for certain defects that could result in physical abnormalities or mental retardation. This testing is most common when the mother is over the age of 35 or there is a family history of physical or mental abnormalities.
A genetic disease may be apparent when the child is born or may appear later as the child develops. Genetic testing can help diagnose these diseases. Couples who are having difficulty conceiving a child or who have suffered multiple miscarriages may be tested to see if a genetic cause can be identified.
Huntington's disease is an example of a genetic disease that doesn't appear until adulthood. If this disease or another late-onset disease is in a person's family, genetic testing may be able to predict if that person will develop the disease.
Some genetic defects may make a person more susceptible to certain types of cancer. Testing for these defects can help predict a person's risk. Other types of genetic tests help diagnose and predict and monitor the course of certain kinds of cancer, particularly leukemia and lymphoma.
Precautions
A person usually meets with a genetic counselor, a person with a master's degree in genetic counseling or a physician specializing and board certified in genetics (a medical geneticist), before most genetic tests.
The counselor should review the person's family history and medical records and the reason for the test. The counselor should explain the likelihood that the test will detect all possible causes of the disease in question (known as the sensitivity of the test), and the likelihood that the disease will develop if the test is positive (known as the positive predictive value of the test).
Learning about the disease in question, the benefits and risks of both a positive and a negative result, and what treatment choices are available if the result is positive, will help prepare the person undergoing testing. The counselor should make sure the person understands how the test results will affect his or her life, family, and future decisions.
After this discussion, the person should have the opportunity to indicate in writing that he or she gave informed consent to the test, verifying that the counselor provided complete and understandable test information.
Description
Genes and chromosomes
Deoxyribonucleic acid (DNA) is a long molecule made up of two strands of material coiled around each other in a unique double helix structure. This structure was discovered in 1953 by Francis Crick and James Watson.
DNA is found in the nucleus, or center, of most cells (Some cells, such as a red blood cell, don't have a nucleus). Each person's DNA is a unique blueprint, giving instructions for a person's physical traits, such as eye color, hair texture, height, and susceptibility to disease. DNA is organized into structures called chromosomes.
The instructions are contained in DNA's long strands as a code spelled out by pairs of bases, which are four chemicals that make up DNA. The bases occur as pairs because a base on one strand lines up with and is bound to a corresponding base on the other strand. The order of these bases form DNA's code. In each cell, there are 3 billion base pairs.
A grouping of base pairs that give instruction for a specific trait is called a gene. Each gene has an assigned place on a specific chromosome. Each normal cell has 46 chromosomes arranged into 23 pairs. Each parent contributes one chromosome to each pair. The first 22 pairs, called autosomal chromosomes, are assigned a number from 1-22. The last pair are the sex chromosomes and include the X and the Y chromosomes. If a child receives an X chromosome from each parent, the child is female. If a child receives an X from the mother, and a Y from the father, the child is male.
Just as each parent contributes one chromosome to each pair, so each parent contributes one gene to each pair. The pair of genes produces a specific trait in the child. Usually one gene has a stronger influence on the trait than the other gene. The stronger gene is called dominant; the weaker gene, recessive. Two copies of a recessive gene are needed to control a trait while only one copy of a dominant gene is needed.
Types of tests
Genetic disease results from a change, or mutation, in a chromosome or in one or several base pairs in a gene. Several types of genetic tests are available to look for the mutations in genes and chromosomes associated with certain diseases. The cost of genetic tests vary: chromosome studies can cost hundreds of dollars and certain gene studies, thousands. Insurance coverage also varies with the company and the policy. It may take several days or weeks to complete a test.
Direct DNA mutation analysis
Direct DNA mutation analysis examines DNA for specific gene mutations. Some genes contain more than 100,000 bases and a mutation of any one base can make the gene nonfunctional and cause disease. The more mutations possible, the less likely it is for a test to detect all of them. This test is usually done on white blood cells from a person's blood. The test begins by using chemicals to separate DNA from the rest of the cell. Next, the two strands of DNA are separated by heating. Special enzymes (called restriction enzymes) are added to the single strands of DNA and then act like scissors and cut the strands in specific places. The DNA fragments are then sorted by size through a process called electrophoresis. A special piece of DNA, called a probe, is added to the fragments. The probe is designed to bind to specific mutated portions of the gene. When bound to the probe, the mutated portions appear on x-ray film with a distinct banding pattern.
Family linkage studies
Family linkage studies are done to study a disease when a mutated gene's general location on a chromosome is known but its identity is not. These studies are possible when a chromosome marker has been found associated with a disease. Chromosomes contain certain regions that vary in appearance between individuals. These regions are called polymorphisms. If a polymorphism is always present in family members with the same genetic disease, and absent in family members without the disease, it is likely that the gene responsible for the disease is near that polymorphism. The gene mutation can be indirectly detected in family members by looking for the polymorphism.
To look for the polymorphism, DNA is isolated from cells in the same way it is for direct DNA mutation analysis. A probe is added that will detect the large polymorphism on the chromosome. When bound to the probe, this region will appear on x-ray film with a distinct banding pattern. The pattern of banding of a person being tested for the disease is compared to the pattern from a family member affected by the disease.
Linkage studies have disadvantages not found in direct DNA mutation analysis. These studies require multiple family members to participate in the testing. If key family members choose not to participate, the incomplete family history may make testing other members useless. The indirect method of detecting a mutated gene also causes more opportunity for error.
Chromosome analysis
Many genetic diseases and syndromes are caused by structural chromosome abnormalities. To analyze a person's chromosomes, his or her cells are allowed to grow and multiply in the laboratory until they reach a certain stage of growth. The length of growing time varies with the type of cells. Cells from blood and bone marrow take 1-2 days; fetal cells from amniotic fluid take 7-10 days.
When the cells are ready, they are placed on a microscope slide using a technique to make them burst open, spreading their chromosomes. The slides are stained: the stain creates a banding pattern unique to each chromosome. Under a microscope, the chromosomes are counted, identified, and analyzed based on their size, shape, and stained appearance.
Karyotypes of the chromosomes are prepared for further study and to document the results. First, a photograph is taken of the chromosomes from one or more cells as seen through the microscope. Then the chromosomes are cut out and arranged side-by-side with their partner in ascending numerical order, from largest to smallest. The karyotype is done either manually or using a computer attached to the microscope. Chromosome analysis is also called cytogenetics.
Applications
Carrier testing
A person who has a mutated gene associated with a disease is called a carrier. A carrier is a person who is not affected by the mutated gene he or she possesses, but can pass the gene to an offspring. Genetic tests have been developed that tell prospective parents whether or not they are carriers of certain diseases. If one or both of the parents is a carrier, the risk of passing the disease to a child can be predicted.
To predict the risk, it is necessary to know if the gene in question is autosomal or sex-linked. If the gene is carried on any one of chromosomes 1-22, the resulting disease is called an autosomal disease. If the gene is carried on the X or Y chromosome, it is called a sex-linked disease.
Sex-linked diseases, such as the bleeding condition hemophilia, are usually carried on the X (or female) chromosome. A woman who carries a disease-associated mutated gene on one of her X chromosomes, has a 50% chance of passing that gene to her son. A son who inherits that gene will develop the disease because he does not have another normal copy of the gene on a second X chromosome to compensate for the mutated copy.
The risk of passing an autosomal disease to a child depends on whether the gene is dominant or recessive. A prospective parent carrying a dominant gene, has a 50% chance of passing the gene to a child. A child needs to receive only one copy of the mutated gene to be affected by the disease.
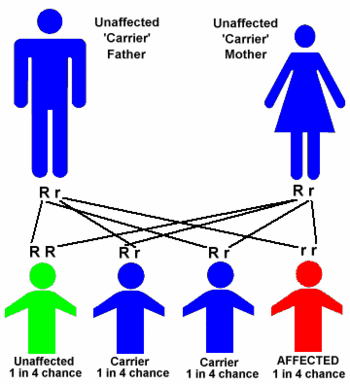
If the gene is recessive, a child needs to receive two copies of the mutated gene, one from each parent, to be affected by the disease. When both prospective parents are carriers, their child has a 25% chance of inheriting two copies of the mutated gene and being affected by the disease; a 50% chance of inheriting one copy of the mutated gene, and being a carrier of the disease but not affected; and a 25% chance of inheriting two normal genes. When only one prospective parent is a carrier, a child has a 50% chance of inheriting one mutated gene and being an unaffected carrier of the disease, and a 50% chance of inheriting two normal genes.
Cystic fibrosis is a disease that affects the lungs and pancreas and is discovered in early childhood. It is the most common autosomal recessive genetic disease found in the caucasian population: 1 in 25 people of Northern European ancestry are carriers of a mutated cystic fibrosis gene. The gene, located on chromosome 7, was identified in 1989.
The gene mutation for cystic fibrosis is detected by a direct DNA test. Over 600 mutations of the cystic fibrosis gene have been found; each of these mutations cause the same disease. Tests are available for the most common mutations. Tests that check for the six most common mutations will detect 85% of carriers for cystic fibrosis. If a person tests negative, it is likely, but not guaranteed that he or she does not have the gene. Both prospective parents must be carriers of the gene to have a child with cystic fibrosis.
Tay-Sachs disease, also autosomal recessive, affects children primarily of Ashkenazi Jewish descent. Children with this disease die between the ages of two and five. This disease was previously detected by looking for a missing enzyme. The mutated gene has now been identified and can be detected using direct DNA mutation analysis.
Presymptomatic testing
Not all genetic diseases show their effect immediately at birth or early in childhood. Although the gene mutation is present at birth, some diseases don't appear until adulthood. If a specific mutated gene responsible for a late-onset disease has been identified, a person from an affected family can be tested before symptoms appear.
Huntington's disease is a fatal autosomal dominant disease. Its symptoms of mental confusion and abnormal body movements don't appear until middle to late adulthood. The chromosome location of the gene responsible for Huntington's chorea was located in 1983 after studying the DNA from a large Venezuelan family affected by the disease. Ten years later the gene was identified. A test is now available to detect the presence of the mutated gene in a person. The presence of the mutated dominant gene means the person will develop the disease.
The specific genetic cause of Alzheimer's disease is not as clear. Although many cases appear to be inherited in an autosomal dominant pattern, many cases exist as single incidents in a family. Like Huntington's, symptoms of mental deterioration first appear in adulthood. Genetic research has found an association between this disease and genes on four different chromosomes. The validity of looking for these genes in a person without symptoms or without family history of the disease is still being studied.
Cancer susceptibility testing
Cancer can result from an inherited mutated gene or a gene that mutated sometime during a person's lifetime. Some genes, called tumor suppressor genes, produce proteins that protect the body from cancer. If one of these genes develops a mutation, it can't produce the protective protein. If the second copy of the gene is normal, its action may be sufficient to continue production, but if that gene later also develops a mutation, the person is vulnerable to cancer. Other genes, called oncogenes, are involved in the normal growth of cells. A mutation in an oncogene can cause too much growth, the beginning of cancer.
Direct DNA tests are currently available to look for gene mutations identified and linked to several kinds of cancer. People with a family history of these cancers are those most likely to be tested. If one of these mutated genes is found, the person is more susceptible to developing the cancer. The likelihood that the person will develop the cancer, even with the mutated gene, is not always known because other genetic and environmental factors are also involved in the development of cancer.
Cancer susceptibility tests are most useful when a positive test result can be followed with clear treatment options. In families with familial polyposis of the colon, testing a child for a mutated APC gene can reveal whether or not the child needs frequent monitoring for the disease. In families with potentially fatal familial medullary thyroid cancer or multiple endocrine neoplasia type 2, finding a mutated RET gene in a child provides the opportunity for that child to have preventive removal of the thyroid gland. In the same way, MSH1 and MSH2 mutations can reveal which members in an affected family are vulnerable to familiar colorectal cancer and would benefit from aggressive monitoring.
In 1994, a mutation linked to early-onset familial breast and ovarian cancer was identified. BRCA1 is located on chromosome 17. Women with a mutated form of this gene have an increased risk of developing breast and ovarian cancer. A second related gene, BRCA2, was later discovered. Located on chromosome 13, it also carries increased risk of breast and ovarian cancer. Although both genes are rare in the general population, they are slightly more common in women of Ashkenazi Jewish descent.
When a woman is found to have a mutation of one of these genes, the likelihood that she will get breast or ovarian cancer increases, but not to 100%. Other genetic and environmental factors influence the outcome.
Testing for these genes is most valuable in families where a mutation has already been found. BRCA1 and BRCA2 are large genes; BRCA1 includes 100,000 bases. More than 120 mutations to this gene have been discovered, but a mutation could occur in any one of the bases. Studies show tests for these genes may miss 30% of existing mutations. The rate of missed mutations, the unknown disease likelihood in spite of a positive result, and the lack of a clear preventive response to a positive result, make the value of this test for the general population uncertain.
Prenatal and postnatal chromosomeanalysis
Chromosome analysis is done on fetal cells primarily when the mother is over the age of 35, has had multiple miscarriages, or a family history of a genetic abnormality. Prenatal testing is done on the fetal cells in amniotic fluid (the fluid surrounding the baby) at 14-16 weeks of pregnancy or from a chorionic villus sampling (from the baby's placenta) at 8-12 weeks. Cells from amniotic fluid grow for 7-10 days before they are ready to be analyzed. Biopsy cells grow faster and can be analyzed sooner.
Chromosome analysis using blood cells is done on a child who is born with or later develops signs of mental retardation or physical malformation. In the older child, chromosome analysis may be done to investigate developmental delays.
Extra or missing chromosomes cause mental and physical abnormalities. A child born with an extra chromosome 21 (trisomy 21) has Down syndrome. An extra chromosome 13 or 18 also produce well known syndromes. A missing X chromosome causes Turner syndrome and an extra X in a male causes Klinefelter syndrome. Other abnormalities are caused by extra or missing pieces of chromosomes. Fragile X syndrome is a sex-linked disease, causing mental retardation in males. The abnormality is recognized by a fragile-looking area at the bottom of the X chromosome.
Chromosome material may also be rearranged, such as the end of chromosome 1 moved to the end of chromosome 3. If no material is added or deleted in the exchange, the person may not be affected. Such an exchange, however, can cause infertility or abnormalities if passed to children.
Evaluation of a man and woman's infertility or repeated miscarriages will include blood studies of both to check for a chromosome structural rearrangement. Many chromosome abnormalities are incompatible with life; babies with these abnormalities often miscarrry during the first trimester. Cells from a baby that died before birth can be studied to look for chromosome abnormalities that may have caused the death.
Cancer diagnosis and prognosis
Certain cancers, particularly leukemia and lymphoma, are associated with changes in chromosomes: extra or missing complete chromosomes, extra or missing portions of chromosomes, or exchanges of material (called translocations) between chromosomes. Studies show that the locations of the chromosome breaks are at locations of tumor suppressor genes or oncogenes.
Chromosome analysis on cells from blood, bone marrow, or solid tumor helps diagnose certain kinds of leukemia and lymphoma and often helps predict how well the person will respond to treatment. After treatment has begun, periodic monitoring of these chromosome changes in the blood and bone marrow gives the physician information as to the effectiveness of the treatment.
A well-known chromosome rearrangement is found in chronic myelogenous leukemia. This leukemia is associated with an exchange of material between chromosomes 9 and 22. The resulting smaller chromosome 22 is called the Philadelphia chromosome.
Preparation
Most tests for genetic diseases of children and adults are done on blood. To collect the 5-10 mL of blood needed, a healthcare worker draws blood from a vein in the inner elbow region. Collection of the sample takes only a few minutes.
Prenatal testing is done either on amniotic fluid or a chorionic villus biopsy. To collect amniotic fluid, a physician performs a procedure called amniocentesis. An ultrasound is done to find the baby's position and an area filled with amniotic fluid. The physician inserts a needle through the woman's skin and the wall of her uterus and withdraws 5-10 mL of amniotic fluid. Placental tissue for a chorionic villus biopsy is taken through the cervix. Each procedures take approximately 30 minutes.
Bone marrow is used for chromosome analysis in a person with leukemia or lymphoma. The person is given local anesthesia. Then the physician inserts a needle through the skin and into the bone (usually the sternum or hip bone). One-half to 2 mL of bone marrow is withdrawn. This procedure takes approximately 30 minutes.
Aftercare
After blood collection the person can feel discomfort or bruising at the puncture site or may become dizzy or faint. Pressure to the puncture site until the bleeding stops reduces bruising. Warm packs to the puncture site relieve discomfort.
Collection of amniotic fluid, chorionic villus biopsy, and bone marrow are all done under a physician's supervision. The person is asked to rest after the procedure and is watched for weakness and signs of bleeding.
Risks
Collection of amniotic fluid and chorionic villus biopsy have the risk of miscarriage, infection, and bleeding; the risks are higher for the biopsy. A woman should tell her physician immediately if she has cramping, bleeding, fluid loss, an increased temperature, or a change in the baby's movement following either of these procedures.
After bone marrow collection, the puncture site may become tender and the person's temperature may rise. These are signs of a possible infection.
Genetic testing involves other nonphysical risks. Many people fear the possible loss of privacy about personal health information. Results of genetic tests may be reported to insurance companies and affect a person's insurability. Some people pay out-of-pocket for genetic tests to avoid this possibility. Laws have been proposed to deal with this problem. Other family members may be affected by the results of a person's genetic test. Privacy of the person tested and the family members affected is a consideration when deciding to have a test and to share the results.
A positive result carries a psychological burden, especially if the test indicates the person will develop a disease, such as Huntington's chorea. The news that a person may be susceptible to a specific kind of cancer, while it may encourage positive preventive measures, may also negatively shadow many decisions and activities.
Normal results
A normal result for chromosome analysis is 46, XX or 46, XY. This means there are 46 chromosomes (including two X chromosomes for a female or one X and one Y for a male) with no structural abnormalities. A normal result for a direct DNA mutation analysis or linkage study is no gene mutation found.
The person should learn from the genetic counselor the likelihood that the test could miss a mutation or abnormality.
Abnormal results
An abnormal chromosome analysis report will include the total number of chromosomes and will identify the abnormality found. Tests for gene mutations will report the mutations found.
Before making decisions based on an abnormal test result, the person should meet again with a genetic counselor to fully understand the meaning of the results, learn what options are available based on the test result, and what are the risks and benefits of each of those options.
Key Terms
- Autosomal disease
- A disease caused by a gene located on chromosomes 1-22.
- Carrier
- A person who has a disease-causing gene.
- Chromosome
- The structures made up of DNA, on which are located the genes.
- DNA (Deoxyribonucleic acid)
- A long molecule made up of two strands of material coiled around each other in unique double helix. DNA contains the blueprint for a person's traits.
- Dominant gene
- A gene, whose presence as a single copy, controls the expression of a trait.
- Gene
- A grouping of base pairs that give instruction for a specific trait.
- Karyotype
- Visual comparison of chromosomes arranged side-by-side with their partner in ascending numerical order, from largest to smallest.
- Mutation
- Any change in the sequence of DNA.
- Positive predictive value (PPV)
- The probability that a person with a positive test result has, or will get, the disease.
- Recessive gene
- A gene that must be present in both copies of the gene pair to control the expression of a trait.
- Sensitivity
- The likelihood that a negative test means the person will not have the disease or a mutation.
- Sex-linked disorder
- A disorder caused by a gene located on a sex chromosome, usually the X chromosome.
Further Reading
For Your Information
Books
- Berg, Paul, and Maxine Singer. Dealing with Genes: The Language of Heredity. Mill Valley, CA: University Science Books, 1992.
- Farkas, Daniel H. DNA Simplified: The Hitchhiker's Guide to DNA. Washington, DC: American Association of Clinical Chemistry Press, 1996.
- Gelehrter, Thomas D. and Francis S. Collins, and David Ginsburg. Principles of Medical Genetics. 2nd ed. Baltimore: Williams and Wilkins, 1998.
- Grody, Wayne W., and Walter W. Noll. "Molecular Diagnosis of Genetic Diseases. In Clinical Diagnosis and Management by Laboratory Methods, edited by John B. Henry. 19th ed. Philadelphia: W. B. Saunders Company, 1996, pp. 1374-1389.
- Motulsky, Arno G., Richard A. King, and Jerome I. Rotter. The Genetic Basis of Common Diseases. New York: Oxford University Press, 1992.
- Mueller, Robert F. and Ian D. Young. Emery's Elements of Medical Genetics 9th ed. Churchill Livingstone, New York and Edinburgh: 1995.
- Watson, James D. The Double Helix. New York: Atheneum, 1968.
Periodicals
- Auxter, Sue. "Genetic Information--What Should be Regulated?" Clinical Laboratory News. (December, 1997): 9-11.
- Biesecker, Barbara Bowles. "Genetic Susceptibility Testing for Breast and Ovarian Cancer: A Progress Report." Journal of the American Medical Women's Association. (Winter, 1997): 22-27.
- Fink, Leslie and Francis S. Collins. "The Human Genome Project: View From the National Institutes of Health." Journal of the American Medical Women's Association. (Winter, 1997): 4-7, 15.
- Holtzman, Neil A., and Michael S. Watson, eds. Promoting Safe and Effective Genetic Testing in the United States. Final Report of the Task Force on Genetic Testing. National Institutes of Health-Department of Energy Working Group on Ethical, Legal, and Social Implications of Human Genome Research, 1997.
- Holtzman, Neil A., Patricia D. Murphy, Michael S. Watson, and Patricia A. Barr. "Predictive Genetic Testing: From Basic Research to Clinical Practice." Science. (October 24, 1997): 602-605.
- Karnes, Pamela S. "Ordering and Interpreting DNA tests." Mayo Clinical Proceedings. (December, 1996): 1192-1195.
- Malone, Kathleen E, Janet R. Daling, Jennifer D. Thompson, Cecilia A. O'Brien, Leigh V. Francisco, and Elaine A. Ostrander. "BRCA1 Mutations and Breast Cancer in the General Population." Journal of the American Medical Association. (March 25, 1998): 922-929.
- McKinnon, Wendy C., Bonnie J. Baty, Robin L. Bennett, Monica Magee, Whitney A. Neufeld-Kaiser, Kathyrn F. Peters, Jill C. Sawyer, and Katherine A. Schneider. "Predisposition Genetic Testing for Late-Onset Disorders in Adults: A Position Paper of the National Society of Genetic Counselors." Journal of the American Medical Association. (October 15, 1997): 1217-1221.
- Newman, Beth, Hua Mu, Lesley M. Butler, Robert C. Millikan, Patricia G. Moorman, and Mary-Claire King. "Frequency of Breast Cancer Attributable to BRCA1 in a Population-Based Series of American Women." Journal of the American Medical Association. (March 25, 1998): 915-921.
- Ponder, Bruce. "Genetic Testing for Cancer Risk." Science. (November 7, 1997): 1050-1054.
- Roses, Allen. "Genetic Testing for Alzheimer Disease. Practical and Ethical Issues." Archives of Neurology. (October, 1997): 1226-1229.
- Whittaker, Lori. "Clinical Applications of Genetic Testing: Implications for the Family Physician." American Family Physician. (May, 1996): 2077-2084.
- Wisecarver, James. "The ABCs of DNA." Laboratory Medicine. (January, 1997): 48-52.
- Yablonsky, Terri. "Genetic Testing Helps Patients and Researchers Predict the Future." Laboratory Medicine. (May, 1997): 316-321.
- Yablonsky, Terri. "Unlocking the Secrets to Disease. Genetic Tests Usher in a New Era in Medicine." Laboratory Medicine. (April, 1997): 252-256.
Organizations
- Alliance of Genetic Support Groups. 4301 Connecticut Avenue NW, Ste. 404, Washington, DC. 20008-2304. http://204.141.0.149/geneticalliance/.
- American College of Medical Genetics. 9650 Rockville Pike, Bethesda, MD 20814-3998. (301) 571-1825. http://www.faseb.org/genetics/acmg/acmgmenu.htm.
- American Society of Human Genetics. 9650 Rockville Pike, Bethesda, MD 20814. (301) 571-1825. http://www.faseb.org/genetics/ashg/ashgmenu.htm.
- Centers for Disease Control. Office of Genetics and Disease Prevention. 4770 Buford Highway NE, Atlanta, GA. 30341-3724. (770) 488-3235. http://www.cdc.gov/genetics/.
- The March of Dimes. 1275 Manaroneck Ave., White Plains, NY 10605. (914) 428-7100. http://www.modimes.org.
- The National Human Genome Research Institute.The National Institutes of Health. 9000 Rockville Pike, Bethesda, MD 20892. (301) 496-2433. http://www.nhgri.nih.gov.
- The National Society of Genetic Counselors. 233 Canterbury Dr., Wallingford, PA 19086-6617. (610) 872-7608. http://members.aol.com/nsgcweb/nsgchome.htm.
Other
- Blazing a Genetic Trail. Online genetic tutorial. http://www.hhmi.org/GeneticTrail/.
- The Gene Letter. Online newsletter. http://www.geneletter.org.
- Online Mendelian Inheritance in Man. Online genetic testing information sponsored by National Center for Biotechnology Information. http://www.ncbi.nlm.nih.gov/Omim/.
- Understanding Gene Testing. Online brochure produced by the U.S. Department of Health and Human Services. http://www.gene.com/ae/AE/AEPC/NIH/index.html.
Gale Encyclopedia of Medicine. Gale Research, 1999.