Epidemiological research is needed to complement new findings in genetics.
Tuberous sclerosis is a dominantly inherited syndrome of high penetrance characterised pathologically by the presence of hamartomas in multiple organ systems. The clinical features of epilepsy, learning difficulties, and skin signs are well known, but recent epidemiological and genetic research has begun to reveal the complexity of the condition.
Compared with many other genetic diseases tuberous sclerosis is common. The two most recent and highest estimates of its prevalence in the United Kingdom have been 3.7 per 100 000 population in the west of Scotland and 3.8 per 100 000 population in Wessex.[1 2] A recent capture-recapture analysis of the Wessex data suggests that this survey failed to identify about half the prevalent cases. The total population prevalence may therefore be as high as 8-9 per 100 000, which means that many people with tuberous sclerosis are receiving neither specialist medical supervision nor genetic counselling.[3]
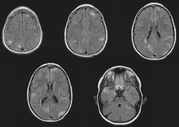
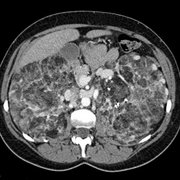
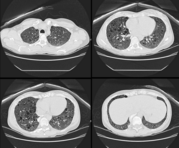
We know now that over half the affected individuals have normal intelligence and a quarter do not have fits. At least 60% of cases represent new mutations. Hamartomatous lesions can develop in almost any organ except skeletal muscle.[1] When they develop in the kidney (cysts, angiomyolipomas) and brain (giant cell astrocytomas) they are potentially life threatening.
Recent advances in molecular genetics have shed some light on the inheritance and pathophysiology of tuberous sclerosis. Linkage studies have shown unequivocal evidence for loci on 9q34 (TSC1) and 16p 13 (TSC2).[4] About half the families informative for linkage studies appear to be linked to TSC1 and half to TSC2. The protein encoded by the TSC2 gene, "tuberin," contains 1807 amino acids[5] and that encoded by the TSC1 gene, "hamartin," 1164 amino acids.[6] Both TSC1 and TSC2 may function as tumour suppressor genes, which, when damaged, allow the proliferation of hamartomas throughout the body. Currently the strongest evidence for this hypothesis comes from studies that show that some of the hamartomas in patients show loss of heterozygosity at 16p13 or at 9q34.[7-8] The loss of heterozygosity implies that an individual with tuberous sclerosis inherits or acquires through mutation a deletion in one copy of the gene but develops lesions only when there is a somatic mutation in the other copy. This "two hit" mechanism was first proposed by Knudson in 1971 to explain the pathogenesis of retinoblastoma but has since been found in other malignant tumours and neurocutaneous syndromes--for example, neurofibromatosis and Von Hippel Lindau disease.[9]
The theory that the genes involved are tumour suppressor genes is also supported by recent research in the Eker rat--an animal model of tuberous sclerosis. The Eker rat has a mutation in the rat homologue of the TSC2 gene and suffers from dominantly inherited renal cell carcinoma and subependymal and subcortical hamartomas. Reintroduction of a wild type TSC2 gene suppresses the development of renal tumours.[10] Search for sequence homologies at the protein level has revealed a region of similarity between tuberin and the GTPase activating protein GAP3.[11] The GTPases are known to be involved in regulating cell proliferation and differentiation, and tuberin may possibly have a role in mediating this activity.
The genetic heterogeneity of tuberous sclerosis raises the question whether the clinical syndrome produced by the two genes is the same. In some conditions, neurofibromatosis for instance, genetic heterogeneity helps explain clinically distinct forms of the disease. All the complications of tuberous sclerosis have been seen both in individuals linked to TSC1 and to TSC2, and the severity of the disease can vary greatly within the same family. Straightforward genotype-phenotype correlations are therefore unlikely. There may, however, be subtle differences in the phenotype produced by the two genes or by specific types of mutations within the genes. Already some evidence from case series suggests that mutations in TSC2 may be more severe than in TSC1.[12] Detailed genotype-phenotype correlation, using a population based sample of patients, should help clarify these points and may, in future, allow clinicians to make more confident predictions about the course of the disease in affected individuals.
Population based studies are also needed to answer questions about the potentially lethal complications in the renal and central nervous systems. The availability of sophisticated imaging techniques means that the lesions produced by this condition are now more clearly visualised and more often detected. For example, renal cysts and angiomyolipomas (hamartomas made up of blood vessels, smooth muscle, and fat) are often found in patients with tuberous sclerosis. Occasionally they may bleed or compress healthy renal tissue but usually they remain asymptomatic. We need to learn how to identify those lesions that will cause problems later. Current recommendations for aggressive intervention are based only on findings from small case series and may not be justified.[13] Only long term follow up of a population based sample of patients with tuberous sclerosis will establish which lesions are likely to become symptomatic and when, if at all, clinical intervention is best timed.
[1] Webb DW, Fryer AE, Osborne JP. Morbidity associated with tuberous sclerosis: a population study. Der Med Child Neurol 1996;38:146-55.
[2] Sampson JR, Scahill SJ, Stephenson JBP, Mann L, Connor JM. Genetic aspects of tuberous sclerosis in the West of Scotland. J Med Genet 1989;26:28-31.
[3] O'Callaghan FJK, Shiell AW, Osborne JP, Martyn CN. Capture-recapture analysis to estimate the prevalence of tuberous sclerosis. Lancet 1998;351:1490.
[4] Povey S, Burley MW, Attwood J, Benham F, Hunt D, Jeremiah SJ, et al. Two loci for tuberous sclerosis: one on 9q34 and one on 16p13. Ann Hum Genet 1994;58:107-27.
[5] European chromosome 16 TS consortium. Cell 1993;75:1305-15.
[6] Van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997;277:805-8.
[7] Green A, Johnson P, Yates J. Loss of heterozygosity on chromosome 16p13.3 in hamartomas from tuberous sclerosis patients. Nat Genet 1994;6:193-6.
[8] Green A, Johnson P, Yates J. The tuberous sclerosis gene on chromosome 9q34 acts as a growth suppressor. Hum Mol Genet 1994;3:1833-4.
[9] Knudson AG. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 1971;68:820-3.
[10] Kobayashi T, Mitani H, Takahashi R, Hirabayashi M, Ueda M, Tamura H, et al. Transgenic rescue from embryonic lethality and renal carcinogenesis in the Eker rat model by introduction of a wild-type TSC2 gene. Proc Natl Acad Sci USA 1997;94:3990-3.
[11] Mashewar MM, Cheadle JP, Jones AC, Myring J, Fryer AE, Harris PC, et al. The GAP-related domain of tuberin, the product of the TSC2 gene, is a target for mis-sense mutations in tuberous sclerosis. Hum Mol Genet 1997;6:1991-6.
[12] Jones AC, Daniells CE, Snell RG, Tachataki M, Idziaszczyk SA, Krawczak M, et al. Molecular genetic and phenotypic analysis reveals differences between TSC1 and TSC2 associated familial and sporadic tuberous sclerosis. Hum Mol Genet 1997;6:2155-61.
[13] Van Baal JG, Smits NJ, Keeman JN, Lindhout D, Verhoef S. The evolution of renal angiomyolipomas in patients with tuberous sclerosis. J Urol 1994;152:35-8.
Finbar J K O'Callaghan Wellcome Trust training fellow in clinical epidemiology
Bath Unit for Research in Paediatrics, Royal United Hospital, Bath BA1 3NH
COPYRIGHT 1999 British Medical Association
COPYRIGHT 2000 Gale Group