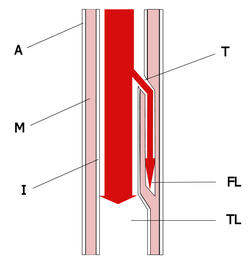
Study objective: To clarify the clinical significance of pleural effusion in the clinical course of acute aortic dissection (AAD).
Design: Retrospective clinical series.
Setting: A university hospital.
Patients: Fifty-five patients strongly suspected of having AAD because of severe chest or back pain. Patients with obvious ischemic heart disease, lung disease, and pleural disease were excluded.
Interventions: An additional diagnosis of pleural effusion was made when evident by CT.
Measurements and results: Pleural effusion was detected in 42 of 48 patients (88%) with AAD (mean [+ or -] SD age, 65 [+ or -] 12 years; 35 men and 13 women), but in only 1 of 7 patients (14%) who proved not to have AAD (mean age, 74 [+ or -] 10 years; 6 men and 1 woman). Effusion appeared at a mean of 4.5 days after onset of dissection. Thoracentesis performed in six patients showed a bloody effusion in three patients and an exudative effusion in three patients. In the six AAD patients without pleural effusion, four patients underwent surgery within 3 days after onset of dissection. In patients with AAD, effusion was demonstrated on the first CT after hospital admission in 18 patients, and was bilateral in 32 patients. WBC count in blood, serum C-reactive protein concentration, and body temperature were higher in patients with effusion (13,400 [+ or -] 3,600/[micro]L, 18.4 [+ or -] 11.5 mg/dL, and 38.2 [+ or -] 0.7 [degrees] C) than in those without effusion (10,300 [+ or -] 2,900/[micro]L, 4.5 [+ or -] 4.2 mg/dL, and 37.0 [+ or -] 1.0 [degrees] C, respectively).
Conclusions: Pleural effusion occurs frequently in patients with AAD, often in association with inflammatory reactions.
Key words: acute aortic dissection; CT; inflammatory reaction; pathogenesis; pleural effusion
Abbreviations: AAD = acute aortic dissection; IL = interleukin
**********
Pleural effusion occurs in various diseases and is an important cause of respiratory dysfunction. Even when not present on initial chest radiography and CT, pleural effusion may appear in patients with acute aortic dissection (AAD), sometimes several days after the onset of dissection. In rare cases, pleural effusion or hemothorax, rather than chest or back pain, is the first sign of AAD. (1-2) The pathogenesis of pleural effusion has been investigated in inflammatory conditions, including tuberculosis, other bacterial and viral infections, and trauma, as well as in neoplasia. (3-11) However, few reports have considered the frequency and pathogenesis of pleural effusion occurring during the clinical course of AAD. (12-14) The aim of this study was to clarify the clinical significance of pleural effusion in patients with AAD, and to investigate the relationship of these effusions to associated clinical events including inflammatory reactions. We evaluated CT and other findings in patients with presumptive diagnoses of AAD.
MATERIALS AND METHODS
Of 1,580 patients admitted to the ICU at our hospital between January 1994 and August 2000, a total of 55 patients strongly suspected of having AAD were studied retrospectively. Forty-eight patients were confirmed to have AAD; chronic aortic dissection was diagnosed in 3 other patients; aneurysm with no dissection was diagnosed in 2 patients; pleuritis was diagnosed in 1 patient; and pain of undefined origin was diagnosed in 1 patient (Fig 1). The diagnosis of AAD was made based on typical chest and back pain and findings on chest radiography, CT, and aortography. CT was performed every 3 to 5 days to reassess the aortic dissection for possible extension. (15) Extension of aortic dissection, appearance of complicating pulmonary disorders, and pleural effusion on serial CT were evaluated by multiple intensivists and radiologists. Radiographic measurement of the pleural effusion was not performed. When the effusion was massive and compromised respiratory function, thoracentesis was performed to evacuate the effusion, and laboratory analysis of pleural fluid was carried out. We sought to determine how frequently and when in the course of AAD pleural effusion occurred. Relationships were evaluated between pleural effusion and various clinical features, including age, gender, site of aortic dissection according to DeBakey et al (16) and Stanford classifications, (17) inflammatory markers (body temperature, WBC count, and C-reactive protein), and clinical outcome. Body temperature, WBC count, and C-reactive protein ordinarily were measured daily for at least 14 days after hospital admission. However, in patients undergoing surgery, reassessment of pleural effusions and inflammatory markers were discontinued after surgery.
[FIGURE 1 OMITTED]
Values are expressed as the mean [+ or -] SD. Statistical analyses were performed by one-way analysis of variance and the [chi] test. A p value < 0.05 was chosen to indicate statistical significance.
RESULTS
Characteristics of Patients
Clinical findings in 48 patients with AAD and 7 patients who proved not to have AAD are presented in Table 1. Differences in age and gender ratio between patients with AAD and those with other diagnoses did not achieve statistical significance. In the group with AAD, 11 patients underwent surgery; 8 patients had Stanford type-A dissections with cardiac and/or cerebral damage, and 3 patients had type-B dissections with impending rupture. The presence of pleural effusion was not an indication for surgery. Twelve patients died during hospitalization, including some deaths in the postoperative period. Of patients without AAD, no patients underwent surgery and two patients died. One patient with AAD was transferred to another hospital 49 days after onset of AAD.
Occurrence of Pleural Effusion
Pleural effusion was detected at various times after hospital admission in 42 of 48 patients (87.5%) with AAD, but in only 1 of 7 patients (14.3%) without AAD. Pleural effusion appeared 4.5 [+ or -] 3.9 days after the onset of AAD (range, 1 to 15 days; Fig 2). Effusion was seen on hospital day i in 18 patients. However, 3 of these patients were admitted to our hospital [greater than or equal to] 2 days after the onset of AAD, so pleural effusion was detected on the first day of dissection in only 15 patients. None had massive hemothorax following rupture of an aneurysm. Pleural effusion resolved before discharge from the hospital (42.2 [+ or -] 20.1 days after onset of dissection) in 22 patients with AAD. In six other patients with AAD, the effusion persisted at discharge from the hospital. In 14 patients with AAD, reevaluations of the pleural effusion were interrupted by death in 8 patients, surgery in 5 patients, and transfer to another hospital in i patient. Characteristics of the 48 patients with AAD with or without pleural effusion are shown in Table 2. No significant difference was noted in age, gender ratio, or category of dissection (Stanford and DeBakey classification). Of the six patients with AAD but no pleural effusion, four patients underwent surgery within 3 days after onset of AAD; two of these patients died after surgery.
[FIGURE 2 OMITTED]
Characteristics of Pleural Effusion
In patients with AAD, effusion was bilateral in 31 patients (73.8%) and left sided in 11 other patients (26.2%). Thoracentesis was performed in six patients because of a compromised respiratory state from a massive pleural effusion. The effusion was bloody in three patients and exudative in three patients (Table 3).
Relationship Between Effusion and Inflammatory Reaction
Inflammatory responses, including maximum WBC count, maximum body temperature, and maximum serum concentration of C-reactive protein, are shown in Figure 3. The WBC count of patients with AAD and pleural effusion was 13,359 [+ or -] 3,588/[micro]L, somewhat higher than in patients with AAD without pleural effusion (10,294 [+ or -] 2,863/[micro]L; difference not significant) and higher than patients without AAD (9,803 [+ or -] 2,136/[micro]L; p < 0.05). Body temperature was 38.2 [+ or -] 0.7 [degrees] C in AAD patients with pleural effusion, 37.0 [+ or -] 1.0 [degrees] C in AAD patients without pleural effusion, and 37.2 [+ or -] 0.7 [degrees] C in the other patients, being significantly higher in AAD dissection patients with pleural effusion than in those without effusion. C-reactive protein was 18.4 [+ or -] 11.5 mg/dL in AAD patients with pleural effusion, significantly higher than in AAD without pleural effusion (4.5 [+ or -] 4.2 mg/dL) or in other patients (5.6 [+ or -] 4.4 mg/dL).
[FIGURE 3 OMITTED]
DISCUSSION
Pleural effusion was demonstrated by CT in most of our patients with AAD (87.5%). Four of the six patients with no occurrence of pleural effusion became candidates for surgery within 2 days after dissection onset. Given that the average time of appearance of pleural effusion on CT was 4.5 days after the onset of AAD, pleural effusion might have developed had surgery not been performed. Hasegawa et al (18) found pleural effusions in eight of nine AAD patients with respiratory failure. In our study, we investigated the occurrence of pleural effusion in patients with AAD without regard to respiratory failure, and pleural effusion still was frequent.
Other authors have found effusion to be less common. Maffei et al (12) reported finding pleural effusion by CT in only 4 of 44 patients with treated AAD; the acute period of aortic dissection was not studied. Our patients were followed up for > 1 month after onset of AAD. Of the 42 patients with pleural effusion, effusion resolved during the month following onset in 22 patients. Eight of the remaining 20 patients died in the hospital, 5 patients underwent surgery, and 1 patient was transferred to another hospital before confirmation of disappearance of the effusion. Pleural effusion persisted in only six patients at discharge from the hospital.
Mechanisms underlying occurrence of pleural effusion have been investigated in cases of infectious disease and of neoplasm. Participation of inflammatory cytokines such as interleukin (IL)-6, IL-8, IL-10, vascular endothelial growth factor, and tumor necrosis factor-[alpha] has received considerable recent attention. (3-11) While cytokine participation in pleural effusion development in patients with AAD is not clear, some investigators have reported possible associations with inflammatory reactions. Hasegawa et al (18) suggested that leucocytosis, high body temperature, thrombocytosis, and elevation of IL-8 were related to AAD and pleural effusion. The results of our study support their suggestion. Schattner et al,19 Veyssier-Belot et al, (20) Giladi et al, (21) and Murray et al (22) reported occurrence of pleural effusion and prolonged fever in some of their patients with chronic aortic dissection.
In rupture of AAD, hemothorax or bloody pleural effusion typically are seen, though occurrence of painless hemorrhagic pleural effusion in patients with AAD also has been reported by several investigators. (1-2) In our study, thoracentesis in six patients demonstrated hemorrhagic effusion in three patients and a nonhemorrhagic exudate in three patients.
When Teasell and Dittmer (23) reported various complications of long-term bed rest, atelectasis and pneumonia were frequent. Cuvelier and Riquet (24) suggested that pleural effusion can occur secondarily to atelectasis. Bed rest is prescribed for patients with AAD to prevent extension of aortic dissection and to control BP. Pleural effusion therefore might have complicated bed rest in some of our patients. However, in 18 patients, the effusion was present on the day of dissection onset; on average, effusions appeared 4.5 days after onset. Effusion apparently resulted from long-term bed rest in only a few of our patients.
Mattison et al (25) reported the frequency of pleural effusion and underlying diseases in an ICU. In their report, effusion was seen in 62% of patients; underlying diseases were congestive heart failure (35%), atelectasis (23%), pneumonia (11%), and various others (31%), including liver dysfunction, cancer, pancreatitis, uremia, and empyema. Pleural effusion associated with AAD was not included in their cases. Notably, these authors studied only 100 patients overall. In our study, 1,580 patients treated in an ICU were evaluated, yielding 48 confirmed cases of dissection.
In conclusion, pleural effusion was frequent in patients with AAD (87.5%) but rare in patients strongly suspected of having AAD who proved to have a different condition. Occurrence of pleural effusion followed onset of AAD by mean of 4.5 days. Pleural effusion demonstrated by serial CT was a common finding in patients with AAD, and also is recognized for altered mental status, ECG changes, aortic insufficiency, loss of pulses, and intractable pain. Evaluation for pleural effusion is highly important in the diagnosis and treatment of aortic dissection and other diseases with chest pain.
REFERENCES
(1) Little S, Johnson J, Moon BY, et al. Painless left hemorrhagic pleural effusion: an unusual presentation of dissecting ascending aortic aneurysm. Chest 1999; 116:1478-1480
(2) Gandelman G, Barzilay N, Krupsky M, et al. Left pleural hemorrhagic effusion: a presenting sign of thoracic aortic dissecting aneurysm. Chest 1994; 106:636-638
(3) Verheul HM, Hoekman K, Jorna AS, et al. Targeting vascular endothelial growth factor blockade: ascites and pleural effusion formation. Oncologist 2000; 5(Suppl 1): 45-50
(4) Yamaguchi T, Kimura H, Yokota S, et al. Effect of IL-6 elevation in malignant pleural effusion on hyperfibrinogenemia in lung cancer patients. Jpn J Clin Oncol 2000; 30:53-58
(5) Aoki Y, Tosato G, Nambu Y, et al. Detection of vascular endothelial growth factor in AIDS-related primary effusion lymphomas [letter]. Blood 2000; 95:1109-1110
(6) Miller EJ, Kajikawa O, Pueblitz S, et al. Chemokine involvement in tetracycline-induced pleuritis. Eur Respir J 1999; 14:1387-1393
(7) Zebrowski BK, Yano S, Liu W, et al. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res 1999; 5:3364-3368
(8) Cheng D, Rodriguez RM, Perkett EA, et al. Vascular endothelial growth factor in pleural fluid. Chest 1999; 116:760-765
(9) Weissflog D, Kroegel C, Luttmann W, et al. Leukocyte infiltration and secretion of cytokines in pleural drainage fluid after thoracic surgery: impaired cytokine response in malignancy and postoperative complications. Chest 1999; 115: 1604-1610
(10) Pace E, Gjomarkaj M, Melis M, et al. Interleukin-8 induces lymphocyte chemotaxis into the pleural space: role of pleural macrophages. Am J Respir Crit Care Med 1999; 159(5 pt 1):1592-1599
(11) Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 1999; 85:178-187
(12) Maffei S, Baroni M, Terrazzi M, et al. Ambulatory follow-up of aortic dissection: comparison between computed tomography and biplane transesophageal echocardiography. Int J Card Imaging 1996; 12:105-111
(13) Langin T, Kelkel PE, Villemot D. Left hemothorax, a complication of a dissecting aneurysm of the descending thoracic aorta [in French]. Rev Mal Respir 1994; 11:74-76
(14) Enomoto S, Miyamoto T, Shimada I, et al. Studies on acute surgical therapy for DeBakey III aortic dissection: especially on the CT images and the operative indication [in Japanese]. Kyobu Geka 1992; 45:1152-1155
(15) Habscheid W. Imaging methods in intensive care [in German]. Bildgebung 1996; 63:4-21
(16) DeBakey ME, Henly WS, Cooley DA, et al. surgical management of dissecting aneurysm of the aorta. J Thorac Cardiovasc Surg 1965; 49:130-149
(17) Miller DC, Stinson EB, Oyer PE, et al. Operative treatment of aortic dissections: experience with 125 patients over a sixteen-year period. J Thorac Cardiovasc Surg 1979; 78:365-382
(18) Hasegawa Y, Ishikawa S, Ohtaki A, et al. Impaired lung oxygenation in acute aortic dissection. J Cardiovasc Surg (Torino) 1999; 40:191-195
(19) Schattner A, Klepfish A, Caspi A. Chronic aortic dissection presenting as a prolonged febrile disease and arterial embolization. Chest 1996; 110:1111-1114
(20) Veyssier-Belot C, de Gennes C, Papo T, et al. An unknown cause of prolonged fever: apropos of 6 cases of chronic aortic dissection [in French]. Rev Med Interne 1998; 19:704-708
(21) Giladi M, Pines A, Averbuch M, et al. Aortic dissection manifested as fever of unknown origin. Cardiology 1991; 78:78-80
(22) Murray HW, Mann JJ, Genecin A, et al. Fever with dissecting aneurysm of the aorta. Am J Med 1976; 61:140-144
(23) Teasell R, Dittmer DK. Complications of immobilization and bed rest: Part 2. Other complications. Can Fam Physician 1993; 39:1440-1446
(24) Cuvelier A, Riquet M. Management. of acute pleural diseases in intensive care units [in French]. Rev Mai Respir 1999; 16:789-797
(25) Mattison LE, Coppage L, Alderman DF, et al. Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest 1997; 111:1018-1023
* From the Intensive Care Unit (Drs. Hata, Imaizumi, Ohara, Ohba, and Shinada), Chiba Hokusoh Hospital, Nippon Medical School, Chiba; and Department of Internal Medicine (Drs. Tanaka and Takano), Nippon Medical School, Tokyo, Japan. The work was performed at Chiba Hokusoh Hospital.
Manuscript received February 13, 2001; revision accepted August 29, 2001.
Correspondence to: Noritake Hata, MD, Intensive Care Unit, Chiba Hokusoh Hospital, Nippon Medical School, 1715 Kamagari, Inbamura, Inbagun, Chiba 270-1694, Japan; e-mail: hatan@ nms.ac.jp
COPYRIGHT 2002 American College of Chest Physicians
COPYRIGHT 2002 Gale Group