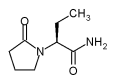
ORLANDO, FLA. -- The antiepilepsy drug levetiracetam, approved in December by the Food and Drug Administration, is an effective add-on treatment for partial-onset seizures, Dr. S. D. Shorvon reported at the annual meeting of the American Epilepsy Society.
Pooled data from 904 adult patients in three double-blind, placebo-controlled trials of levetiracetam as add-on therapy showed that at all doses studied, levetiracetam was significantly better than placebo for controlling seizures in patients who had not responded adequately to their previous therapy, said Dr. Shorvon of the Institute of Neurology at University College of London.
At 3,000 mg/day of levetiracetam, 41% of patients achieved at least a 50% reduction in seizure frequency within 12-14 weeks of treatment. At 2,000 mg/day, 32% of patients had at least a 50% reduction in seizure frequency, and at 1,000 mg, 28% of patients had at least a 50% reduction in seizure frequency during the study period.
A comparable reduction in seizure frequency was seen in only 13% of patients taking placebo, he said.
Overall, 6.3% of the patients who were taking levetiracetam achieved seizure-free status during the trials, compared with 0.4% of patients on placebo. A dose-response effect was seen, with 8% of those in the 3,000 mg/day groups and 4% of those in the 1,000 mg/day groups becoming seizure free, Dr. Shorvon noted.
Levetiracetam was safe at all dosages studied. Four side effects were reported, including somnolence, asthenia, dizziness, and infection; none of these occurred in more than 5% of the patients in any group, he said.
Withdrawal from the study as a result of side effects occurred in 11% of the patients in the levetiracetam group, compared with 8% of the patients in the placebo group.
"This is a well-tolerated drug, with only a few side effects with remarkably infrequent incidence rates, and there are no idiosyncratic effects to date," Dr. Shorvon said.
Preliminary Data On Levetiracetam In Children
Levetiracetam may be safe and effective in children with partial-onset seizures, an open-label study has found.
Of 23 children who were evaluated during a 4-week baseline period, then again after 8 weeks of treatment with levetiracetam, 12 patients (52%) had at least a 50% reduction in seizure frequency, Dr. Tracy Glauser reported at the meeting.
Of the 12 children who responded to levetiracetam, 5 (22%) had at least a 75% reduction in seizure frequency and 2 (9%) became seizure free, said Dr. Glauser of Children's Hospital Medical Center, Cincinnati.
The children, aged 6-12, were on no more than one other antiepilepsy medication. Levetiracetam was given as add-on therapy following the baseline phase, beginning at 10 mg/kg per day and titrated up to the target dose of 40 mg/kg per day, he said.
One child had a 270% increase in seizure frequency at the end of the 8-week treatment phase. That increase appeared to be the result of a low baseline seizure rate, rather than to a medication effect, Dr. Glauser noted.
All of the patients had at least one adverse event, with the most common being headache and infection. Somnolence, anorexia, and nervousness also occurred, he said.
The adverse effects were similar to those that have been seen with other antiepileptic drugs. All of the side effects were rated by the patients and care givers as mild to moderate.
These data are promising, Dr. Glauser commented, noting that a double-blind, controlled trial of levetiracetam to further evaluate safety and efficacy in children is now underway.
COPYRIGHT 2000 International Medical News Group
COPYRIGHT 2001 Gale Group