Abstract
Central adenoid cystic carcinomas are rare malignancies that are believed to arise in ectopic salivary gland tissue within the maxilla or mandible. We describe the diagnosis and treatment of a central adenoid cystic carcinoma in a 54-year-old man, which we believe was a recurrence of an earlier growth that had not been completely excised. We also present a review of the literature.
Introduction
Adenoid cystic carcinomas in general are well known to otolaryngologists. Less well known are central adenoid cystic carcinomas. These tumors are pathologically identical to the more common adenoid cystic carcinomas of the major and minor salivary glands, but they arise from within the mandible or maxilla and can extend to the more usual locations. In this report, we describe a case that we believe represents a recurrent central adenoid cystic carcinoma.
Case report
A 54-year-old black man had a 3-month history of a slowly enlarging, painless mass in his parasymphyseal region. Six years earlier, an otolaryngologist had removed a lesion that involved the mandibular gingiva, but the records of that resection, specifically the pathologic margins, were not available. The patient had a remote history of tobacco use (one pack of cigarettes per day for 5 years), and he denied alcohol use. He also denied dyspnea, dysphagia, odynophagia, and weight loss.
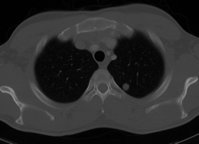
On examination, there was an 8 x 10-cm mass fixed to the mandible in the right parasymphyseal region. The firm, submucosal lesion extended into the floor of the mouth and abutted the lingual aspect of the mandible. There was a palpable 1.5 x 1.5-cm, level II cervical lymph node located ipsilateral to the mass. The patient was edentulous. The remainder of the exam was unremarkable. Computed tomography (CT) was obtained (figure 1), and a biopsy of the lesion was performed in the clinic (figure 2).
After a thorough discussion of the findings, our patient underwent a modified radical neck dissection and hemimandibulectomy as described later in this report.
Discussion
Adenoid cystic carcinoma was originally described by Lorain and Laboulbene in 1853. In 1859, Billroth coined the term cylindroma to describe the pathologic entity we now call adenoid cystic carcinoma. Among the first to use the latter term was Spies in 1930. Until the 1940s, the tumor was thought to be a benign variant of the mixed salivary gland tumor. In 1943, Dockerty and Mayo emphasized the malignant nature of this tumor. For a detailed review of these and other historical aspects of adenoid cystic carcinoma, the reader is referred to the excellent review by S tell. [1]
Adenoid cystic carcinoma is the most common minor salivary gland malignancy, comprising 42% of all such cancers. [2] It is thought to arise from the intercalated ducts of mucus-secreting glands. [1] The most common location of adenoid cystic carcinoma is the posterior hard palate, generally in the area of the greater palatine foramen. The tumor demonstrates a propensity for perineural growth and a high incidence of local recurrence. Less well known, but possibly more important with regard to bloodborne metastasis, is its proclivity for intravascular growth. Longterm followup is essential because these tumors are notorious for their late and persistent recurrences. Distant metastases--most commonly to the lung, brain, and bone--occur in nearly 70% of patients by the time of death. [1] Together, these characteristics account for its ominous prognosis.
There are no universally agreed-upon predisposing factors for the development of adenoid cystic carcinoma, and there seems to be no familial tendency. There is a very slight female preponderance. Patients typically present with a slowly enlarging mass that, because of its indolent growth pattern, can be quite large. The mass is usually painless, although bone invasion or perineural spread can cause pain or hypoesthesia. Most adenoid cystic carcinomas are submucosal and appear as smooth, domed swellings without overlying ulceration. They are unencapsulated but frequently well circumscribed; this appearance is misleading because these lesions have an insidiously infiltrative growth pattern. The incidence of cervical lymphatic metastasis is low: about 8% at presentation and 7% later. [3]
Histologically, there are three recognizable patterns of growth, representing a spectrum of extremes of differentiation: solid, cribriform, and tubuloductal. The solidgrowth architecture portends an extremely poor prognosis in adenoid cystic carcinoma. It is the exception to the indolent growth pattern and usually demonstrates a highly aggressive clinical course, with early and more frequent recurrence (nearly 100%), metastasis, and death within 3 to 4 years of diagnosis. On the other end of the spectrum is the tubuloductal architecture, which is associated with the more-typical indolent growth rate and the lowest rate of recurrence at 59%. Between these extremes lies the most common pattern, the cribriform architecture, which is associated with a recurrence rate of 89%. [4] Batsakis et al have proposed a grading system that considers cytoarchitecture as well as perineural invasion, mitotic rate, stromal changes, necrosis, and the degree to which the border is well-circumscribed. [5]
CT and magnetic resonance imaging are useful in surgical planning, especially with regard to the submucosal extent and perineural spread, which can be difficult to assess clinically. The lesions are generally poorly defined and have infiltrative margins. The center of the lesion, as was the case with our patient, is usually lowdensity on CT, a characteristic that can help differentiate it from a primary squamous cell carcinoma. [6]
The tumor in our patient had its radiographic epicenter within the bone of the mandible. This generated a discussion among the authors as to whether or not it represented a primary central bony adenoid cystic carcinoma of the mandible.
Ectopic salivary gland tissue has been observed in several head and neck locations, including the cervical skin, middle ear, mastoid, maxilla, and mandible. Although rare, salivary gland malignancies can arise in such ectopic salivary gland tissue. Brookstone and Huvos reviewed the relevant literature and found 137 such cases arising in the maxilla and mandible; adenoid cystic carcinoma was the second most common histologic subtype (23 cases), following mucoepidermoid carcinoma (81 cases). [7] They found that swelling and pain were the two most common presenting complaints for central salivary gland tumors involving the jaws. Thirty percent of these cases arose in association with an odontogenic cyst or a recent history of tooth extraction. The pathogenesis of these tumors is controversial, but several authors have proposed that they involve a metaplastic transformation of the mucus-secreting cells commonly found in the epithelial linings of dentigerous cysts and odontogenic epithelium. [7-9] Primary central adenoid cystic carcinomas have a significantly higher rate of metastasis than do mucoepidermoid carcinomas (50 and 9%, respectively). [7]
The treatment of adenoid cystic carcinoma involves surgical resection with wide margins. The surgeon must pay particular attention to obtaining clear margins around regional nerves. The surgical ablation must be individualized because radically mutilating surgery does not appear to improve the prognosis in highly aggressive tumors. Neck dissection should he performed in those patients who have clinical or radiologic evidence of cervical lymphatic metastasis. Prophylactic neck dissection is generally not recommended. [2] Postoperative radiation therapy enhances local and regional control in adenoid cystic carcinoma. Radical surgery followed by postoperative radiation therapy results in 5- and 10-year survival rates of 77 and 57%, respectively. [10]
Ultimately, we determined that the mass in our patient was a primary central adenoid cystic carcinoma and that the patient's previous surgery was an incomplete local excision of a presumed gingival adenoid cystic carcinoma. Our conclusion was supported by the growth pattern on CT. Our patient underwent a modified radical neck dissection and hemimandibulectomy, including reconstruction with a free fibula graft, which provided an excellent functional and cosmetic result. The margins were free of disease, and only the one clinically positive neck node was pathologically positive. Postoperative radiation therapy was administered to the primary site and to the ipsilateral neck. The patient is free of disease at 4.5 years followup.
This case illustrates two key facts regarding the diagnosis and treatment of adenoid cystic carcinoma. First, central salivary gland tumors should be considered in the differential diagnosis of aggressive lesions in the maxilla and mandible. Second, long-term followup is essential regardless of the site because of the tumor's propensity for late recurrence and metastasis.
From the Division of Otolaryngology--Head and Neck Surgery, University of North Carolina School of Medicine, Chapel Hill.
References
(1.) Stell PM. Adenoid cystic carcinoma. Clin Otolaryngol 1986:11:267-91.
(2.) Gluckman JL, Barrord J. Nonsquamous cell tumors of the minor salivary glands. Otolaryngol Clin North Am 1986;19:497-505.
(3.) Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg 1974;128:512-20.
(4.) Perzin KH, Gullane, P, Clairmont AC. Adenoid cystic carcinomas arising in salivary glands: A correlation of histologic features and clinical course. Cancer 1978;42:265-82.
(5.) Batsakis JG, Luna MA, el-Naggar A. Histopathologic grading of salivary gland neoplasms: III. Adenoid cystic carcinomas. Ann Otol Rhinol Laryngol 1990;99:1007-9.
(6.) de Kerviler E, Bely N, Laccourreye O, et al. The aryepiglottic fold as a rare location of adenoid cystic carcinoma. AJNR Am J Neuroradiol 1995;16:1375-7.
(7.) Brookstone MS, Huvos AG. Central salivary gland tumors of the maxilla and mandible: A clinicopathologic study of 11 cases with an analysis of the literature. J Oral Maxillofac Surg 1992;50:229-36.
(8.) Eversole LR, Sabes WR, Rovin S. Aggressive growth and neoplastic potential of odontogenic cysts: With special reference to central epidermoid and mucoepidermoid carcinomas. Cancer 1975;35:270-82.
(9.) Browne RM. Metaplasia and degeneration in odontogenic cysts in man. J Oral Pathol 1972;1:145-58.
(10.) Black KM, Fitzpatrick PJ, Palmer JA. Adenoid cystic carcinoma of the salivary glands. Can J Surg 1980;23:32-5.
COPYRIGHT 2000 Medquest Communications, Inc.
COPYRIGHT 2000 Gale Group