SUMMARY
The Federal District of Brazil (DF) lies within the Cerrado biome, where open shrubland (savannas) is interspersed with riverside gallery forests and permanent swamps (veredas). Trypanosoma cruzi-infected native triatomines occur in the area, but the enzootic transmission of trypanosomatids remains poorly characterized. A parasitological survey involving sylvatic triatomines (166 Rhodnius neglectus collected from Mauritia flexuosa palms) and small mammals (98 marsupials and 70 rodents, totaling 18 species) was conducted in 18 sites (mainly gallery forests and veredas) of the DR Parasites were isolated, morphologically identified, and characterized by PCR of nuclear (mini-exon gene) and kinctoplast DNA (kDNA). Six R. neglectus, seven Didelphis albiventris and one Akodon cursor were infected by trypanosomes; wild reservoir infection is documented for the first time in the DF. kDNA PCR detected T. cruzi in five r. neglectus and mini-cxon gene PCR revealed T. cruzi I in isolates from D. albiventris Parasites infecting one bug yielded T. neglectus KP1+ kDNA amplicons. In spite of the occurrence of T. cruzi-infected D. albiventris (an important wild and peridomestic reservoir) and R. negectus (a secondary vector displaying synanthropic behavior), a low-risk of human Chagas disease transmission could be expected in the DF, considering the low prevalence infection recorded in this work. The detection of T. rangeli KP1+ associated with R. neglectus in the DF widens the known range of this parasite in Brazil and reinforces the hypothesis of adaptation of T. rangeli populations (KP1+ and KP1-) to distinct evolutionary Rhodnius lineages.
KEYWORDS: Trypanosoma cruzi; T. rangeli; Didelphis albiventris; Rhodnius; Enzootic transmission; Federal District, Brazil.
INTRODUCTION
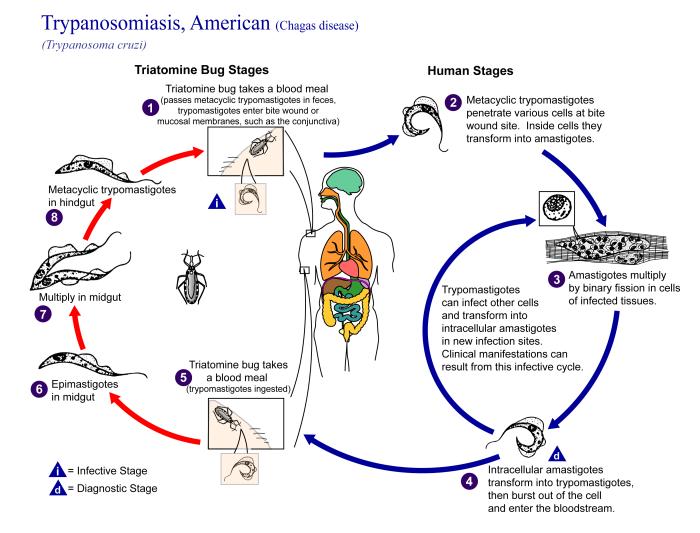
American trypanosomiasis (Chagas disease) is a parasitic zoonosis endemic to the Americas. Trypanosoma (Schizotrypanum) cruzi, a hemoflagellate transmitted by triatomine bugs, is the etiological agent. Over 200 species/subspecies of mammals and 120 triatomine species are known to be susceptible to infection by T. cruzi. Dogs, opossums, rodents, and armadillos act as major reservoirs in human-related environments2,11.
T. cruzi is a remarkably diverse organism. Parasite populations were classified into three major groups (zymodemes Z1, Z2 and Z3) on the grounds of allozyme profiles27,28,30. Extensive isozymic characterization led to further subdivision into up to 43 discrete units41. Later on, molecular markers allowed for the distinction of two major evolutionary clades, and led to the definition of two major groups, T. cruzi I and II (TcI and TcII hereafter)5,10,25. TcI predominates in Amazonian enzootic cycles and in domestic transmission cycles north of the Amazon. A primary association of TcI with didelphid marsupials and Rhodnius vectors, common in Amazonian palm tree habitats, has been proposed. TcII is the main agent of human Chagas disease throughout southern South America, where Triatoina infestans is the primary vector. This parasite lineage may have evolved in terrestrial ecotopes (shared by armadillos, rodents, and several Triatomini), and possibly spread to human habitats when T. infesfans became domestic17,31. However, TcII also appears to be prevalent among non-human primates and Philander opossums33. The extensive intraspecific diversity in T. cruzi probably results from a combination of predominant clonality and nuclear hybridization events18, and has fueled hypotheses linking distinct parasite genotypes with the heterogeneous clinical epidemiology of Chagas disease25,31.
Trypanosoma (Herpetosoma) rangeli also infects mammals and triatomines in Central and South America. Although it causes no disease to humans, it can obscure parasitological and immunological diagnosis of T. cruzi infection in areas where both parasites co-exist sharing hosts and vectors19,21. The cnzootic cycles of this parasite in central Brazil remain however poorly studied35,36.
Molecular studies based on kDNA minicircles and nuclear DNA sequence variation (mini-exon gene) revealed that two populations of T. rangeli with distinct genetic and biological traits are apparently transmitted by different vector species within the genus Rhodnins21,44. These populations can be distinguished by the presence or absence of a conserved, 165-basepair (bp) region in the kinctoplast minicircle, and were accordingly named KP1+ and KP1-.
T. cruzi-infected triatomines species like Panstronglus megistus (BURMEISTER, 1835), Triatoma pseudomaculata (CORRÊA & ESPÍNOLA, 1964), Triatoma sordida (STAL, 1839) and Rhodnius neglectus (LENT, 1954) occurred in the Federal District of Brazil (DF) but the enzootic transmission of trypanosomatids remains poorly characterized. In this paper we report results from a parasitological survey showing that both T. cruzi and T. rangeli circulate in enzootic cycles in the Cerrado of central Brazil. Parasites were isolated and characterized using morphological and molecular techniques. The dynamics of these enzootic transmission cycles and their epidemiological implications are discussed.
MATERIALS AND METHODS
Study areas: The Federal District of Brazil (DF: 15°30'-16°03' S and 47°25'-48°12' W) lies on a high plateau (1000 m above sea level) dominated by the Cerrado biome within the State of Goiás. The Cerrado is composed of seasonally dry, open shrubland (savannas) interspersed with gallery forests and permanent swamp areas (veredas) where Mauritia flexuosa palm trees (locally known as buriti) are dominant37. The study region has a mean annual rainfall of 1545 mm with a dry season (precipitation
Triatomine collection and parasite detection: Triatomines were collected in eight veredas from M. flexuosa palm tree crowns; manual captures and live-baited adhesive traps were combined (see GURGELGONÇALVES et al.23). Twenty-live M. flexuosa were sampled in each vereda performing two hundred palm trees that were grouped in three kinds of landscapes: wild, rural and periurban accordingly to the adjacent area from the vereda (wild - gallery forests and savannas, rural - cattle and crops, pcriurhan - houses and streets in a perimeter of one kilometer)22. The criteria of inclusion were also accessibility and entrance permission by the government and farmers. Classical parasitological7 and molecular techniques (see below) were used for detecting natural infection by trypanosomatids.
Capture and identification of small manimaLs: Small mammals were captured in thirteen areas between years 2000 and 2003; our survey was linked to a research project on small mammal community ecology in the Cerrado biome32. Gallery forests of different hydrographic basins were studied preferentially. One vereda and one area of open shrubland were also sampled (Table 1). One 290 m-long trapping line with 30 capture stations evenly spaced (10 m) was set in each area. Capture stations consisted of two Sherman traps, one at ground level and one on the understory (1-2 m above ground). Half of the traps were large-sized (11 x 12.5 x 37 cm) and the rest small-sized (8 x 9 x 23 cm). Both trap types were set on the ground in alternate capture stations. Two or three Tomahawk traps ( 17 x 17 x 52 cm), set on the ground in random sites, were added to each trapping line. A mixture of peanut cream, corn meal, banana, and sardine was used as bait. In one area (Riacho Fundo, RF) only Young traps (12 x 15 x 40 cm) were used. They were regularly distributed on a line of 840 m, totaling 42 groundlevel stations separated by 20 m. The small mammal captures were performed in two periods of four nights: one in dry season and the other in rainy season, performing a capture effort of 500 traps/night in each of 12 area studied. In addition, in the Riacho Fundo (RF) area the captures were done in the beginning of wet season with a continuous capture effort of 210 traps/night per week. Mammals were identified after PALMA32.
Xenodiagnosis, in vitro isolation, and morphological identification of parasites: Laboratory-reared R. neglectus (six to ten 4th-5th instar nymphs by test) and Dipetalogaster maxima (1st instar nymphs) were used for xenodiagnosis on 168 mammals. Three bugs were used with mammals weighting less than 100 g. The mammals were anaesthetized using 50 mg/kg of cctamine (Ketamina®) mixed with 0.025 mg/kg of xylasinc (Rompum®). The hindgut, feces, haemolymph, and salivary glands of the bugs were examined four weeks later using standard parasitological protocols7. Xenodiagnosis-positive trialomines were washed with White solution and the abdomen dissected30. Feces and hindgut were removed, submerged in Gentamycin/5-Fluorocytosine solution (100 µg/ml), homogenized, and inoculated in two or three tubes containing Blood Agar medium (DIFCO) with a liquid supernatant (medium RPMI-1640). Culture tubes were maintained at 28 °C and weekly examined for two months. Isolates were cryoprescrvecl in liquid nitrogen for future studies. Smears containing triatomine feces or material obtained from culture tubes were stained with Giemsa®buffered solution. Morphological differences between T. cruzi and T. rangeli were assessed following well-established procedures for parasite identification7. Field-collected triatomines were subjected to identical treatment.
DNA extraction and PCR amplification:
Opossum samples: Parasite DNA was extracted using the phenolchloroform method and ethanol/sodium acetate precipitation from three types of samples derived from xenodiagnosis-positive triatomines: concentrated culture samples (CCS hereafter), culture samples impregnated on filter paper (CFP), and bug fecal samples impregnated on filler paper (BFP). Standard PCRs (95 °C for five minutes plus 35 cycles of denaturation [95 °C, one min], primer annealing [60 °C, one min] and extension [72 °C, one min]) were performed in a Minicycler® (MJ Research, France).
Initially a PCR using primers S35 and S36 was carried out to differentially detect kDNA from T. cruzi and T. rangeli20 in samples from seven D. albiventris (see Table 2): two CCS (SV1 and RF6), five CFP (RF2, RF4, RF5, RF8, and RFl 1), and four BFP (RF2, RF4, RF5, and RF11). T. cruzi-positive samples were characterized as TcI or TcII using duplex PCR with primers TCC, TC1 and TC215; four CFP (RF2, RF4, RF5, and RF8), three BFP (RF2, RF4, and RF11), and two CCS (SVl and RF6) were tested.
Samples from sylvatic Rhodnius neglectus: DNA was extracted (as above) directly from hindgut samples of 33 bugs collected in palms of Sctc Veredas (SV). Detection and characterization of T. rangeli (as KP1+ or KP1-) was carried out in Colombia by one of us (JC Carranza) using duplex PCR with primers S35, S36 and KP1L44.
All PCR products were resolved by electrophoresis on 6% polyacrylamide gel stained with silver nitrate. Interpretation and analysis of banding patterns followed VALLEJO et al.44.
RESULTS
Small mammals infected by Trypanosoma cruzi in gallery forests: 98 marsupials and 70 rodents, belonging to 17 genera and 18 species, were captured and examined by xenodiagnosis in different areas of the DF (Tables 2 and 3). Eight of these small mammals were infected by trypanosomatids: seven marsupials (Didelphis albiventris) and one rodent (Akodon cursor). Examination of salivary glands and haemolymph of triatomines fed on 74 of the mammals (rodents and marsupials) did not reveal evidence of infection by T. rangeli.
Seven trypanosomatid populations obtained by xenodiagnosis from D. albiventris (one captured in SV and six captured in RF) were isolated in vitro. Twenty-eight out of 37 culture tubes inoculated were positive (75.6%), while nine isolates failed to grow. Epimastigotes and mctacyclic trypomastigotes with size, shape, and a large rod-shaped kinctoplast characteristic of T. cruzi were observed in positive culture samples. T. cruzi-like trypanosomes isolated from A. cursor by xenodiagnosis did not grow in Blood Agar cultures, precluding further characterization.
Rhodnius neglectus infected by Trypanosoma spp in veredas of the DF: Natural infection of R. neglectus by Trypanosoma spp was detected in two veredas sited within rural farms. An overall infection rate of 3.6% was obtained (Table 4). SV 9.1% out of 33 triatomines examined were infected by trypanosomes. In Tabatinga-106 (TA) infection index was 8.1% (3/37). In SV two bugs presented 71 cruzi-likc trypanosomes in their hindgut contents, and one bug presented molecular evidence of 71 rangeli infection (see below). Trypanosomes from the three bugs infected in TA were morphologically indistinguishable from 71 cruzi.
Molecular characterization of the parasites:
Parasites from Didelphis albiventris: Three of the samples tested by PCR with primers S35-S36 were positive for 71 cruzi kDNA, yielding a 330-bp amplicon: one BPP (RF11) and two CCS (SV1 and RP6). None of these samples were positive for 71 rangeli (Fig. 2). Infection by TcI (presence of a 350-bp amplicon) was revealed by PCR with primers TCC, TC1 and TC2 in six samples: one BPP (RPl 1), two CCS (SVl and RP6) and three CFP (RF2, RF4 and RF8) (Fig. 3).
Parasites from Rhodnius neglectus: PCR with primers S35-S36 revealed 71 cruzi infection in five sylvatic bugs: three from TA and two from SV. 71 rangeli (characterized as KP1+ by the presence of a 165-bp amplicon after duplex PCR with primers S35, S36 and KP1L) was detected in one bug from SV (Fig. 4).
DISCUSSION
The Cerrado is a complex and diverse biome of central Brazil, where several native Triatominac species arc present. Some of them, including R. neglectus, may act as secondary vectors of human Chagas disease. Control of synanthropic populations of T. infestans (an introduced species) is reducing Chagas disease incidence rates throughout the region, and public health strategies now concentrate on the monitoring of transmission by autochthonous vectors11. The circulation of trypanosomatid parasites in wild cycles in the Cerrado remains however poorly investigated. The characterization of the dynamics of these cycles (including parasites, vectors and mammal reservoirs) is of foremost importance for the definition of vigilance schemes; our research aims at contributing to current efforts on that direction.
The overall prevalence of T. cruzi infection in potential reservoirs was low (4.8%) in our study area; it was however unevenly distributed among sites. Less than 10 mammals were studied in seven out of the 10 sites where no infection was detected; only a wider sampling could allow for the hypothesis of small, contained foci of transmission being suitably tested. Taking only into account sites with larger samples (> 10 mammals) the prevalence rate rises to 6.8%, with didelphid marsupials accounting for 87.5% of infections. Regardless of infection rates, this constitutes the first documented record of the enzootic circulation of T. cruzi among small mammals in the DF.
As expected, the opossum D. albiventris played a significant role as reservoir of T. cruzi. Several behavioral (synanthropism, nomadic habits, use of hollow trees and palms as refuges) and biological traits (two generations/year, life-long T. cruzi infection with long-lasting parasitemia) of this opossum make it a good candidate link between wild and peridomestic cycles of T. cruzi transmission, even in areas where stable, domiciliated vector colonies are uncommon13,14,34,39,40. The relatively low specific infection rate we found (14%) rises to 33% when considering only the gallery forest where most opossums were captured (RF, 18 individuals). Rates from 19% to 92% have been reported for Didelphis spp from different regions of South and Central America2,36,40.
Over 50 species of rodents have been found infected by T. cruzi in the Americas (22 in Brazil), but they seem to play a minor role in the maintenance of wild cycles in central Brazil. Infection rates of 0.1% (1/963) and 0.3% (2/722) have been reported from Goiás26 and São Paulo16, respectively. Only one rodent (Akodon cursor) was infected in our sample (n = 70), resulting in rates of 1.4% for all rodents and 14.3% for A. cursor (1/7). Contamination of culture tubes precluded further characterization of these irypanosomatid parasites. T. cruzi infection rales of 18.4% have been reported for A. cursor2.
R. neglectus, common in Cerrado palm trees (mainly Acrocomia, Attalea and Mauritia), is considered as a potential secondary vector of human Chagas disease throughout its wide range in central Brazil (Bahia, DF, Goiás, Mato Grosso, Minas Gerais, Paraná, and São Paulo), where it invades and occasionally colonizes artificial ecotopes6,11. The presence of adult bugs in human dwellings and peridomestic habitats has been reported in the Slates of São Paulo, Goiás and Minas Gerais, with average T. cruzi infection rates of 3.4%2. R. neglectus is frequently found in houses in several districls of Goiás, where 1.5% of the bugs were infected38, and has been sporadically captured in houses and peridomestic habitais in the DP41, with unknown infection rates. Results presented here show that R. neglectus also acts as a vector in enzootic transmission cycles of T. cruzi in veredas of the Cerrado. The low infection rate we report (3.6%) is probably related to the preference of palm tree-living bug populations for avian blood, but illustrates a potential risk that cannot be ignored by public health officials.
Molecular characterisation of parasites isolated from D. albiventris captured in the DF revealed TcI. Particular phylogenetic groups of T. cruzi seem to preferentially infect certain reservoir hosts. There is evidence suggesting thai TcII (but not TcI) causes severe, parasitemic disease to rodents, while D. inarsupialis eliminate TcII and retain TcI24. Field sludies show that TcI is often associated with marsupials (mainly Didelphis spp.), while TcII is more commonly isolated from placental mammals, including humans31. However, enzootic cycles involving TcII and marsupials (Philander frenata) have also been described, even in areas where TcI was isolated from sympatric Didelphis33. The relationships among wild cycles and between these and domestic ones are extremely complex, but a common trait is that opossums seem to play a key role in transmission dynamics13,15,24.
Studies conducted in Brazil showed that TcI was present in only a small proportion of acute chagasic patients, while TcII was isolated from the vast majority of chronic cases. TcI is believed to frequently result in asymptomatic chronic infections. However, mortality rates of acute patients from the Amazon are similar to those reported for areas where TcII prevails. Parasites characterized as TcI circulate in endemic areas of Venezuela where the major chronic manifestations of the disease seem to be present in low percentages28. Recent reviews5,10,25 argue that TcI does not sustain long-lasting infections and is an opportunistic parasite generally unable to cause severe chronic disease to humans, but the authors judiciously recommend specific research to support this idea. Indeed, clinical-epidemiological data from areas where TcI prevails in domestic cycles (northern South America, Central America, and Mexico) show that the infection is a significant cause of disease and suffering3,4. Transmission must therefore be combated disregarding the parasite strains locally involved.
We have shown that R. neglectus is also involved in the transmission of T. rangeli KP1+, adding support to the hypothesis of adaptation (and perhaps co-evolution) of two parasite populations (KP1+ and KP1-) to distinct evolutionary Rhodnius lineages (the pictipes-pallescens-colombiensis-ecuadoriensis group with KP1- and the prolixus-mbustus-nasulus-neglectus-domesticus group with KP1+)21,44. In Brazil, T. rangeli has been found infecting wild mammals and vectors in the Amazon8,9,29, R. domesticus in Bahia1, wild rodents (Echimys dasythrix) in Santa Catarina42, sylvatic R. neglecius from Tocantins12, and, recently, D. albiventris and R. neglectus in Minas Gerais35,36. We present here molecular evidence that T. rangeli infects R. neglectus from M. flexuosa palm trees in the DF, widening the known geographic range of this parasite in Brazil and warning about possible false-positive results of diagnostic tests for T. cruzi infection.
CONCLUSIONS
In spite of the sympatric occurrence of D. albiventris (an important wild and peridomestic T. cruzi reservoir) and R. neglectus (a secondary vector able of invading and sometimes colonizing human-related environments), both infected by T. cruzi, a low-risk of human Chagas disease transmission could be expected in the DF, considering the low level of infection prevalence amongst reservoirs and vectors observed in this work. But our results lend firm support to the idea that continuous epidemiological-entomological surveillance is crucial in areas where control of strictly domestic triatomines is yielding satisfactory results but enzootic transmission persists11.
Inhabited rural areas (such as SV, where infected vectors and reservoirs are present) will deserve special attention, particularly whenever invasion and/or colonization of synanthropic ecotopes by native, secondary vectors are reported. The study of enzootic T. cruzi cycles in untamed environments (such as RF, a protected ecological reserve) may provide researchers with a much-needed better insight on natural transmission dynamics within sylvatic foci. Ultimately, it is the elucidation of the ecological interactions between pathogens, reservoirs, vectors, humans, and their shared environment that will provide the keys for sustainable control and surveillance of anthropozoonotic infectious diseases whose eradication will remain unfeasible.
RESUMO
Transmissão cnzootica dc Trypanosoma cruzi e T. rangeli no Distrito Federal, Brasil
O Distrilo Federal (DF) do Brasil está localizado no bioma Cerrado, urn complexo de fisionomias savânicas incluindo matas de galeria e campos úmidos permanentes (veredas). Triatomíneos silvestres infectados por Trypanosoma cruzi ocorrem na área, mas a transmissão enzoótica de tripanossomatídeos permanece insufficientemente caractcrizada. Um estudo parasitológico envolvendo triatomíneos silvestres (166 Rhodmus neglectus coletados em palmeiras da espécie Mauritia flexuosa) e pequenos mamíferas (98 marsupiais c 70 roedores, totalizando 18 espécies) foi conduzido em 18 áreas, principalmente matas dc galeria e veredas. Os parasitas foram isolados, identificados morfologicamente e caracterizados por PCR do DNA do cinetoplasto (kDNA) e núcleo (gene mini-exon). Seis R. neglectus, sete Didelphis albivenlris e um Akodon cursor estavam infectados por tripanossomatídeos; a infecção cm reservatórios silvestres é documentada pela primeira vez no DF. O PCR do kDNA detectou T. cruzi em cinco K. negleclus e? PCR do gene mini-exon revelou T. cruzi I nos isolados de D. albiventris. Um dos insetos mostrou estar iniectado por T. rangeli KPl+. Apesar da ocorrência de D. albiventris (um importante reservatório silvcstre e peridoméstico) e R. neglectus (um vctor secundário capaz de invadir domicílios) infectados por T. cruzi, urn baixo risco de transmissão da doença de Chagas humana séria esperado no DF, considerando a baixa prevalência da infecção apresentada neste trabalho. A evidência molecular apresentada neste trabalho confirma a circulação dc T. rangeli KP1+ com R. neglectus coino vctor, amplia a distribuição geogrâfica deste parasita no Brasil e reforça a hipótesc de adaptação de populações de T. rangeli (KP1+ e KP1-) a diferentes Hnhagens evolutivas de espécies de Rhodnius.
ACKNOWLEDGEMENTS
We are deeply grateful to Fábio, Rafaël, Samuel, Rinara, Fernanda, and Wanessa for their assistance in fieldwork. Special thanks are due to Dr GA Vallejo for generously providing the primers used in molecular analyses and Marcclo Lima Reis for providing permission and technical support to work in Santuário da Vida Silvestre of Riacho Fundo. We also thanks to the anonymous reviewers of this paper.
REFERENCES
1. BARRETT, T.V. & OLIVEIRA, T.S. - A trypanosoma indistinguishable from Trypanosoma rangeli in the hacmolymph of Rhodnius domesticus from Brazil. Trans, roy. Soc. trop. Med. Hyg., 71: 445-446, 1977.
2. BARRETTO, M. P. - Epidcmiologia. In: BRENI-R, Z. & ANDRADE, Z.A., ed. Trypanosoina cruzi e docnça de Chagas. Rio de Janeiro, Guanabara Koogan, 1979. p. 89-151.
3. BOSSENO, M.F.; BARNABÉ, C.; MAGALLÓN-GASTÉLUM, E. et al. - Predominance of Trypanosuma cruzi lineage I in Mexico. J. clin. Microbiol., 40: 627-632, 2002.
4. BRENIÉRE, S.F.; BOSSENO, M.F.; TELLERIA, S. et al. - Different behaviour of two Trypanosoma cruzi major clones: transmission and circulation in young Bolivian patients. Exp. Parasit., 89: 285-295, 1998.
5. BUSCAGLIA, C.A. & DI NOIA, J.M. - Trypanosoma cruzi clonal diversity and epidemiology of Chagas disease. Microbes Infect., 5: 419-427, 2003.
6. CARCAVALLO, R.U.; RODRÍGUEZ, M.E.F.; SALVATELLA, R. et al. - Habitats and related fauna. In: CARCAVALLO, R.U.; GALÍNDEZ GIRÓN, I.; JURBERG, J. & LENT, H., ed. Atlas of Chagas disease vectors in Americas. Rio de Janeiro, FIOCRUZ, 1998. v. 2, p. 561-600.
7. CUBA CUBA, C. A. - Revisión de los aspectos biológicos y diagnósticos del Trypanosoma (Herpetosoma) rangeli. Rev. Soc. bras. Mod. trop., 31: 207-220, 1998.
8. D'ALESSANDRO, A.; EBERHARD, M.; de HINCAPIÉ, O. & HALSTEAD, S. - Trypanosoma cruzi and Trypanosoma rangeli in Saimiri sciureus from Bolivia and Saguinus mislax from Brasil. Amer. J. trop. Med. Hyg., 35: 285-289, 1986.
9. DEANE, L.M. - Novo hospedeiro de tripanosomas dos tipos cruzi e rangeli no Estado do Pará. O marsupial Meiacliirops opossum opossum. Rev. bras. Malar., 10: 531-541, 1958.
10. DEVERA, R.; FERNANDES, O. & COURA, J.R. - Should Trypanosoma cruzi be called "cruzi" complex? A review of the parasite diversity and the potential of selecting population after in vilro culturing and mice infection. Mem. Inst. Oswaldo Cruz, 98: 1-12, 2003.
11. DIAS, J.C.P. - Epidemiologia. In: BRENER, Z. & ANDRADE, Z.A., ed. Trypanosoma cruzi e doenca de Chagas. Rio de Janeiro, Guanabara Koogan, 2000. p. 48-54.
12. DIOTAIUTI, L; SILVEIRA, A.C.; ELIAS, M. & STEINDEL, M. - The possibility of occurrence of Trypanosoma rangeli in the State of Tocantins, Brazil. Mem. Inst. Oswaldo Cruz, 87: 451, 1992.
13. DIOTAIUTI, L.A.; PEREIRA, A.S.; LOIOLA, C.F. el at. - Inter-relation of sylvatic and domestic transmission of Trypanosoma cruzi in areas with and without vectorial transmission in Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz, 90: 443-448, 1995.
14. FERNANDES, A.J.; CHIARI, E.; RODRIGUES, R.R.; DIAS, J.C. & ROMANHA, AJ. - The importance of the opossum (Didelphis albivenlris} as a reservoir for Trypanosoma cruzi in Bambui, Minas Gerais State. Mem. Inst. Oswaldo Cruz, 86: 81-85, 1991.
15. FERNANDES, O.; MANGIA, R.H.; LISBOA, C.V. et al. - The complexity of the sylvatic cycle of Trypanosoma cruzi in Rio de Janeiro State (Brazil) revealed by the nontranscribed spacer of the mini-exon gene. Parasitology, 118: 161-166, 1999.
16. FORATTINI, O.P.; JUÁREZ, E.; RABELLO, E.X.; PATTOLI, D. & CORREA, R.R. - Infestaçãao domiciliar por Triatoma infestans e alguns aspectos epidemiologicos da tripanossomi'ase americana em área do Estado de São Paulo, Brasil. Rev. Saúde públ. (S. Paulo), 3: 159-172, 1969.
17. GAUNT, M. & MILES, M. - The ccotopes and evolution of triatomine bugs (Triatominae) and their associated trypanosomes. Mem. Inst. Oswaldo Cruz, 95: 557-565, 2000.
18. GAUNT, M.W.; YEO, M.; FRAME, I.A. el al. - Mechanism of genetic exchange in American trypanosomes. Nature, 421: 936-939, 2003.
19. GRISARD, E.C.; STEINDEL, M.; GUARNERI, A.A. el al. - Characterization of Trypanosoma rangeli strains isolated in Central and South America: an overview. Mem. Inst. Oswaldo Cruz, 94: 203-209, 1999.
20. GUHL, F.; JARAMILLO, C.; CARRANZA, J.C. & VALLEJO, G.A. - Molecular characterization and diagnosis of Ttypanosoma cruzi and Trypanosoma rangeli. Arch, med. Res., 33: 362-370, 2002.
21. GUHL, F. & VALLEJO. O.A. - Tiypaimstuna (Herpelusoma) rangeli Tejera. 1920: an updated review. Mein. Inst. Oswalde Cruz, 98: 435-442, 2003.
22. GURGEL-GONÇALVES, R. - Distribuiçã cspacial (Ic populacções de Trialominac (Hcmipliira: Reduviidae) em |)alniciras da espccie Mauritiajlextwsa e circulação enzoótica de Trypanonoma cruzi e Trypanoaoiua rangeli no Distrito Federal, Brasil. Brasilia, 2003. (Dissertação de Mestrado - Faculdade de Ciências da Saúde da Universidade de Brasília).
23. GURGEL-GONÇALVES, R.; PALMA, A.R.T.; MENEZES, M.N.A.; LEITE, R.N. & CUBA CUBA, C.A. - Sampling Rhodnius negteclus (Trialominae) in Maiitilia flexuosa palm trees (Arecaceae): a field study in the Brazilian Savanna. Med. vet. Ent., 17: 347-349. 2003.
24. JANSEN, A.M.; PINHO, A.P.S.; LISBOA, C.V. et al.-The sylvatic cycle of Trypanosoma cruzi: a still unsolved puzzle. Mem. Inst. Oswalde Cruz, 94: 203-204, 1999.
25. MACEDO, A.M.; MACHADO, C.R.; OLIVEIRA, R.P. & PENA, S.D.J. - Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem. Inst. Oswaldo Cruz, 99: 1-12, 2004.
26. MELLO, D.A. - Aspectos do ciclo silvestre do Trypanosoma cruzi em regiões do Cerrado (Município de Formosa, Goiás). Mem. Inst. Oswaldo Cruz, 76: 227-246, 1981.
27. MILES, M.A.; SOUZA, A.; PÓVOA, M.M. et al. - Isozymic heterogeneity of Trypanosoma cruzi in the first autochthonous patients with Chagas disease in Amazonian Brazil. Nature, 272: 819-821, 1978.
28. MILES, M.A.; CEDILLOS. R.A.; PÓVOA, M.H. el al. - Do radically dissimilar Trypanosoma cruzi strains (zymodcmcs) cause Venezuelan and Brazilian forms of Chagas disease? Lancet, 1: 1338-1340, 1981.
29. MILES, M.A.; ARIAS, J.R.; VALENTE, S.A.S. et al. - Vertebrate hosts and vectors of Tiypanosoina rangelt in the Amazon basin of Brazil. Amer. J. trop. Med. Hyg., 32: 1251-1259, 1983.
30. MILES, M.A.; APT, B.W.; WIDMER. G.; POVOA, M.M. & SCHOEIELD, CJ. - Isoenzyme heterogeneity and numerical taxonomy of Trypanosoma cruzi stocks from Chile. Trans, roy. Soc. trop. Med. Hyg., 78: 526-535, 1984.
31. MILES, M.A.; FELICIANGELI, M.D. & ROJAS DE ARIAS, A. - American trypanosomiasis (Chagas disease) and the role of molecular epidemiology in guiding control strategies. Brit. med. J., 326: 1444-1448, 2003.
32. PALMA, A.R.T. - Estrutura de comunidades de pequenos mamíferos no Cerrado. Brasília, 2003. (Tcse de Doutorado - Instituto de Biologin da Universidade de Brasília).
33. PINHO, A.P.; CUPOLILLO, E.; MANGIA, R.H.; FERNANDES, O. & JANSEN, A.M. - Trypanosoma cruzi in the sylvatic environment: distinct transmission cycles involving two sympatric marsupials. Trans, roy. Soc. trop. Med. Hyg., 94: 509-514, 2000.
34. RABINOVICH, J.; SCHWEIGMANN, N.; YOHAI, V. & WISNIVESKY-COLLI, C. - Probability of Trypanosoma cruzi transmission by Triatoma infestanx (Hemiptera: Reduviidae) to the opossum Didelphis albiventris (Marsupialia: Didelphidae). Amer. J. trop. Med. Hyg., 65: 125-130, 2001.
35. RAMIREZ, L.E.; MACHADO, M.I.; MAYWALD, PG. et al. - Primeira evidância de Trypanosoma rangeli no sudeste do Brasil, região endcmica para doença de Chagas. Rev. Soc. bras. Med. trop., 31: 99-102, 1998.
36. RAMIREZ, L.E.; LAGES-SILVA, E.; ALVARENGA-FRANCO, F. et al. - High prevalence of Trypanosoma rangeli and Trypanosoma cruzi in opossums and triatomids in a formerly-endemic area of Chagas disease in Southeast Brazil. Acta trop. (Basel), 84: 189-198, 2002.
37. RIBEIRO, J.F. & WALTER, B.M.T. - Fitofisionomias do bioma cerrado. In: SANO, S.M. & ALMEIDA, S.P., ed. Cerrado anibicntc c Horn. Planaltina, Embrapa, 1998. p. 89-166.
38. SANTOS, A.H.; ISAC, E.; SILVA, J.L. et al. - Indices de infestação domiciliar e infecção natural pelo Trypanosoma criizi das espécies de triatomineos capturadas no estado de Goiás, no període 1994-1998. In: Congrcsso Brasileiro de Parasitologia, 14, Poços de Caldas, 1999. Resumes, p. 150.
39. SCHWEIGMANN, N.I.; PEITROKOVSKY, S.; BOTTAZZI, V.; CONTI, O. & WISNIVESKY-COLLI, C. - Internetiou between Didelphis albivenlris and Trialoma infestans in relation to Trypanosoma cruzi transmission. Mem. Inst. Oswaldo Cruz, 90: 679-682, 1995.
40. SCHWEICiMANN, N.J.; PEITROKOVSKY, S.; BOTTAZZI, V. a al. - Prevalence of Trypanosoma cruzi infection in opossum (Didelphis albiventris) in Santiago del Estero, Argentina. Rev. Parrainer. Salud pub]., 6: 371-377, 1999.
4L SILVEIRA, A.C.; FEITOSA, V.R. & BORGES, R. - Distribuição de triatomíneos capturados no ambientc domiciliai', no període de 1975/83, Brasil. Rev. bras. Malar., 36: 15-312, 1984.
42. STEINDEL, M.; CARVALHO-PINTO, J.C.; TOMA, U.K. el al. - Trypanosnma rangeli (Tejera, 1920) isolated from a sylvatic rodent (Echimys dasvthrix) in Santa Catarina Island, Santa Catarina State: first report of this trypanosoma in southern Brazil. Mem. last. Oswaldo Cruz, 86: 73-79,1991.
43. TIBAYRENC, M. & AYALA, F.J. - Isoenzyme variability of Trypanosoma cruzi, the agent of Chagas disease: genetic, taxonomy and epidemiological significance. Evolution, 42: 277-292, 1988.
44. VALLEJO, G.A.; GUHL, F.; CARRANZA, J.G. el al. - Parity between kinetoplast DNA and mini exon gene sequences support cither clonal evolution or speciation in Trypanosoma rangelt strains isolated from Rhodnius colombiensis, R. pallescens and R. prolixus in Colombia. Infect. Gen. Evol., 67: 1-7, 2002.
Received: 1 June 2004
Accepted: 14 October 2004
Rodrigo GURGEL-GONÇALVES(1,2), Eduarde Dias RAMALHO(1), Marco Antônio DUARTE(1), Alexandre Ramlo Torre PALMA(2), Fernando ABAD-FRANCH(3), Julio Cesar CARRANZA(4) & César Augusto CUBA CUBA(1)
Research partially funded by CNPq and FINATEC.
(1) Laboratório de Parasilologia Médica e Biologin de Vetores, Área de Patologia, Faculdadc de Medicina, Univcrsidade de Brasília, Brasília DF, Brazil.
(2) Laboratório de Zoologia, Universidade Católica de Brasília, Brasília DF, Brazil.
(3) Centro de Pesquisa Leônidas & Maria Deane, Fiocruz/Amazônia, Manaus, Amazonas, Brazil.
(4) Laboratorio de Investigaciones en Parasitología Tropical, Universidad del Tourna, Pacultad de Ciencias, Ibagué, Tolina. Colombia.
Correspondence to: César Augusto CUBA CUBA, Laboratório de Parasitologia Médica e Biologia de Vetores, Faculdade de Medicina, Universidade de Brasília, Campus Universitário Darcy Ribeiro, Asa Norte, 70910-900 Brasília, Distrito Federal, Brazil. Fax: +55-61-273.3907. E-mail: cuba@unb.br
Copyright Instituto de Medicina Tropical de Sao Paulo Nov/Dec 2004
Provided by ProQuest Information and Learning Company. All rights Reserved