Study objective: Laboratory testing plays a minor role in the assessment of aortic dissection. Its main value is in the exclusion of other diseases. Following an incidental observation, we systematically investigated the relationship between elevated d-dimer levels and acute aortic dissection.
Design: We prospectively tested d-dimer levels in patients with suspected acute aortic dissection (10 patients). In addition, we investigated 14 patients who had received a confirmed diagnosis of thoracic aortic dissection during the previous 5 years, in whom d-dimer testing had been performed for differential diagnosis. Thirty-five patients with acute chest pain of other origin served as a control group.
Setting: Tertiary referral hospital.
Patients: Twelve patients had type A dissection (Stanford classification), and 12 patients had type B. Measurements and results: A d-dimer analysis was performed (Tina-quant assay; Roche Diagnostics; Mannheim, Germany) [normal limit of the assay, 0.5 [micro]g/mL]. The result of the d-dimer test was positive (ie, > 0.5 [micro]g/mL) in all patients (sensitivity of the test, 100%) with a mean value of 9.4 [micro]g/mL and a range of 0.63 to 54.7 [micro]g/mL. The degree of the elevation was correlated to the delay from the onset of symptoms to laboratory testing (mean, 12.6 h; range, 1 to 120 h) and showed a trend to the extent of the dissection, but not to the outcome (14 patients could be discharged; 10 patients died).
Conclusions: Based on our observation, we suggest that testing for d-dimer should be part of the initial assessment of patients with chest pain, especially if aortic dissection is suspected. A negative test result makes the presence of the disease unlikely.
Key words: aorta; coagulation; d-dimer; dissection
Abbreviations: DVT = deep vein thrombosis; PE = pulmonary embolism; TEE = transesophageal echocardiography; TTE = transthoracic echocardiography
**********
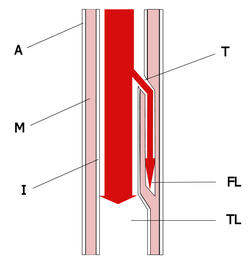
Acute dissection of the thoracic aorta is a severe disease with high mortality rates. In suspected cases, diagnostic speed is of utmost importance. (1) Available noninvasive bedside tests in the emergency department, including physical examination, ECG, chest radiograph, and transthoracic echocardiography (TTE) can provide important clues to the diagnosis in some patients, but they usually are not sufficient to rule out the disease. More accurate tests are semi-invasive (eg, transesophageal echocardiography [TEE]) or are time-consuming and not available at bedside (eg, CT scanning, MRI, and angiography). Until now, laboratory testing has played only a minor role in the assessment of acute aortic dissection, (1) and tests are performed only for the exclusion of other diseases.
MATERIALS AND METHODS
We prospectively tested d-dimer levels in patients with acute aortic dissection (10 patients). In addition, we identified 54 patients who had received this diagnosis in the previous 5 years in our hospital database. In 14 of these patients, a d-dimer test had been performed for differential diagnosis, and the results of those tests were included in our analysis. Thirty-five consecutive patients who were admitted to our cardiology ICU due to acute chest pain of an origin other than aortic dissection served as a control group.
The diagnosis of aortic dissection was confirmed in all patients using standard criteria (1) with TTE, TEE, CT scanning, MRI, angiography, and/or autopsy. We classified the disease according to the systems of Stanford and DeBakey, and by the new differentiation proposed by Svensson et al. (2) In addition, we characterized the extent of the disease by the involvement of the thoracic aorta only or by the additional involvement of the abdominal aorta or even the iliac arteries.
D-dimer analysis was performed (Tina-quant assay; Roche Diagnostics; Mannheim, Germany) with the aid of an automated chemical analysis system (model 704; Hitachi; Tokyo, Japan). This test uses a latex-bound monoclonal antibody that is specific to d-dimer. The reaction is followed at an assay temperature of 37[degrees]C via the increase in turbidity at a wavelength ranging from 700 to 950 nm. The result is available in 15 to 30 min. The test has a lower detectable limit of 0.04 [micro]g/mL. The lower cutoff value for the exclusion of deep vein thrombosis (DVT), which is the main use of the test in our hospital, is 0.5 [micro]g/mL (3).
Data are presented as numbers, percentages, mean [+ or -] SD. The groups were compared by means of the Mann-Whitney U test, analysis of variance, and Spearman correlation, as appropriate. A value of p < 0.05 was considered to be significant.
RESULTS
Patients
Baseline characteristics are shown in Table 1, and dissection types, management, and clinical outcomes in Table 2. The diagnostic procedures were as follows: TTE, 23 patients (96%); TEE, 5 patients (21%); CT scanning, 17 patients (71%); MRI, 4 patients (17%); and angiography, 4 patients (17%). In seven patients with type A dissection, no surgery was performed because of refusal by the surgeon or the patient.
Control Group
The final diagnoses for the 35 consecutive patients with acute chest pain were as follows: acute myocardial infarction, 10 patients; unstable angina, 10 patients; heart failure, 5 patients; atypical chest pain, 5 patients; aortic stenosis, 2 patients; arrhythmias, 2 patients; and pulmonary embolism (PE), 1 patient.
D-dimer Results
D-dimer was positive (ie, a level of > 0.5 [micro]g/mL) in all patients with acute aortic dissection, with a mean value of 9.4 [micro]g/mL and a range of 0.63 to 54.7 [micro]g/mL. Correlations between the extent of the dissection and the absolute d-dimer values are shown in Table 3 and Figure 1. D-dimer values tended to be higher in more extended disease. In addition, there was a statistically significant negative correlation between the absolute values and the time from the onset of symptoms (r = -0.41; p = 0.04). However, we found no relationship between d-dimer levels and the presence or absence of pericardial effusion or in-hospital outcome (differences were not significant for either). D-dimer was positive in 11 control group patients (31%).
[FIGURE 1 OMITTED]
Including the control group into the analysis and maintaining a cutoff value of 0.5 [micro]g/mL for the test, we found a sensitivity of 100%, a specificity of 68.6%, a positive predictive value of 68.6%, and a negative predictive value of 100% for d-dimer in patients with acute aortic dissection.
Only a small minority of the patients had other potential causes of d-dimer elevation as follows: one patient had a stent implanted in an abdominal aortic aneurysm 4 weeks prior to the dissection; one patient received an IM injection of an analgesic due to misdiagnosis of the origin of his chest pain 6 h before the d-dimer test; one patient had previously operated prostatic cancer without overt metastases; and two patients had a history of DVT.
DISCUSSION
Acute dissection is a rare but often catastrophic illness. Early and accurate diagnosis and treatment are crucial for survival. The most common clinical presentation of persons with the disease is severe chest pain. (4) Associated clinical criteria like pulse or BP differentials can be helpful in suspecting the diagnosis, (5) but they are present only in a minority of patients. (6) So, the authors of a multicenter registry (6) concluded that a high clinical index of suspicion is necessary for rapid diagnosis and treatment of acute aortic dissection. This high index of suspicion leads to appropriate testing. Bedside tests like the ECG, chest radiograph, and TTE cannot rule out the diagnosis of acute dissection. (1) More advanced imaging modalities can refute the diagnosis, but they are either semi-invasive (ie, TEE) or time-consuming and are not available in the emergency department setting (ie, CT scanning, MRI, and aortography). Moreover, the accuracy of these tests is dependent on their performance and interpretation by skilled individuals. So, a simple and quick laboratory test to rule out acute dissection would be of great value.
Until now, blood testing has played only a minor role in diagnosing acute aortic dissection. Due to the often large wound surface, elevations of C-reactive protein and lactic acid dehydrogenase can be found in some patients. (7) An immunoassay of smooth muscle myosin heavy chain has been evaluated, showing high sensitivity and specificity, (7) but the test is not widely used today.
D-dimer is a typical degradation product of cross-linked fibrin. D-dimers are detectable at levels of > 0.5 [micro]g/mL fibrinogen equivalent units in nearly all patients with venous thromboembolism. The sensitivity and the negative predictive value of the test for DVT and/or PE are > 90%. (8) However, d-dimer is nonspecific. Elevated d-dimer levels generally can be seen with intravascular activation of the coagulation system and secondary fibrinolysis, in particular in patients with malignancies, (9) disseminated intravascular coagulation, (10) severe infections, complicated renal disease, recent trauma or surgery, and following fibrinolytic therapy. (11) Due to the high negative predictive value, the test can be used for the exclusion of DVT and PE.
A patient with suspected PE (based on chest pain and elevated d-dimer level) who is referred for TTE to rule out pulmonary hypertension turned out to have an aortic dissection. This incidental finding lead us to investigate all patients with dissections. We found that all patients who had experienced aortic dissections in our institution in the previous 5 years in whom the test was performed had elevated d-dimer levels. From a pathophysiologic point of view, this finding can be explained by the activation of the extrinsic pathway of the coagulation cascade at the site of vessel (aortic) wall injury by tissue factor. (12) This fits in with our observation that the extent of the aortic injury, expressed as the anatomic extent of the dissection, correlates with the absolute degree of d-dimer elevation. Our findings seem to extend prior reports of disseminated intravascular coagulation in patients with dissecting aneurysms (13) and in patients with residual dissections after undergoing surgical treatment of type A dissection. (14)
In addition, the time from the beginning of symptoms to d-dimer testing seems to play a role in determining the absolute d-dimer value. The shortest time interval in our study was 1 h, the longest interval was 120 h, and there was a negative correlation between these intervals and the d-dimer concentrations. However, d-dimer values were elevated in all patients with aortic dissections regardless of the time interval. In general, d-dimer levels caused by tissue injury show a trend for gradual decrease within the first few days after experiencing the trauma. In a study of patients treated for acute DVT, d-dimer levels decreased sharply in the first few days of treatment but were still abnormal on day 10. (15) In a recent study, (16) d-dimer levels did not normalize within 14 days in severely traumatized patients. Taken together, these findings may indicate a potential limitation of the diagnostic value of d-dimer testing in the course of the aortic dissection after the first days, but not in the acute setting.
Taking the results from our control group into account, the role of d-dimer testing in patients with suspected acute dissection might resemble its established role in suspected DVT and PE. The high negative predictive value of a negative test result renders the presence of the disease very unlikely. However, the low specificity makes the interpretation of a positive value difficult.
Our study has the following two important limitations: (1) the relatively small patient number due to the low incidence of the disease; and (2) the predominant retrospective character of our findings. Both could be overcome only by a prospective large-scale multicenter study.
CONCLUSION
These data provide evidence that a negative d-dimer test result could be useful in excluding acute thoracic aortic dissection.
REFERENCES
(1) Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection: recommendations of the Task Force on Aortic Dissection, European Society of Cardiology. Eur Heart J 2001; 22:1642-1681
(2) Svensson LG, Labib SB, Eisenhauer AC, et al. Intimal tear without hematoma. Circulation 1999; 99:1331-1336
(3) Dempfle CE, Hafner G, Lestin HG, et al. Multicenter evaluation of Tina-quant D-dimer. J Lab Med 1996; 20:31-37
(4) Spittell PC, Spittell JA Jr, Joyce JW, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc 1993; 68:642-651
(5) Von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med 2000; 160:2977-2982
(6) Hagan PG, Nienaber CA, Isselbacher EM. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000; 283:897-903
(7) Suzuki T, Katoh H, Watanabe M, et al. Novel biochemical diagnostic method for aortic dissection: results of a prospective study using an immunoassay of smooth muscle myosin heavy chain. Circulation 1996; 93:1244-1249
(8) Goldhaber SZ, Simons GR, Elliott CG, et al. Quantitative plasma D-dimer levels among patients undergoing pulmonary angiography for suspected pulmonary embolism. JAMA 1993; 270:2819-2822
(9) Costantini V, Zacharski LR. Fibrin and cancer. Thromb Haemost 1993; 69:406-414
(10) Bick RL. Disseminated intravascular coagulation: objective laboratory diagnostic criteria and guidelines for management. Clin Lab Med 1994; 14:729-768
(11) Kraus M. Fibrin(ogen)-spaltprodukte, D-Dimere. In: Thomas L, ed. Labor und diagnose. 5th ed. Frankfurt/Main, Germany: Books Verlagsgesellschaft mbH, 1998; 648-651
(12) Rapaport SI, Rao LV. The tissue factor pathway: how it has become a "prima ballerina." Thromb Haemost 1995; 74:7-17
(13) McLeod AA, Cuddigan BJ, Mahon WE. Chronic hemorrhagic disorder induced by dissecting aortic aneurysm. Eur Heart J 1980; 1:409-411
(14) Nakajima T, Kin H, Minagawa Y, et al. Coagulopathy associated with residual dissection after surgical treatment of type A aortic dissection. J Vasc Surg 1997; 26:609-615
(15) DVTENOX Study Group. Markers of hemostatic system activation during acute deep venous thrombosis: evolution during the first days of heparin treatment. Thromb Haemost 1993; 70:909-914
(16) Johna S, Cemaj S, O'Callaghan T, et al. Effect of tissue injury on D-dimer levels: a prospective study in trauma patients. Med Sci Monit 2002; 8:CR5-8
* From the Cardiology Department, General Hospital of the Barmherzigen Schwestern, Wels, Austria.
Manuscript received April 11, 2002; revision accepted September 9, 2002.
Correspondence to: Thomas Weber, MD, Second Internal Department/Cardiology, General Hospital of the Barmherzigen Schwestern, Grieskirchnerstrasse 42, A-4600 Wels, Austria; e-mail: webertom@aon.at
COPYRIGHT 2003 American College of Chest Physicians
COPYRIGHT 2003 Gale Group