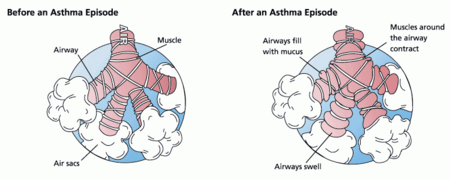
The 1997 guidelines from the National Asthma Education and Prevention Program (NAEPP) (1) noted that inhaled corticosteroid therapy offered multiple benefits in patients with persistent asthma, but some uncertainty remained about its use in certain patients. The NAEPP's recent update (2) of the 1997 guidelines clarifies such treatment issues and should significantly change the way asthma is treated. Part I (3) of this two-part article reviewed diagnosis, monitoring, and prevention of disease progression in patients with asthma. Part II reviews updated recommendations for the treatment of asthma and discusses areas of controversy, including combination therapy and the use of antibiotics for asthma exacerbations.
Inhaled Corticosteroids
The previous NAEPP guidelines (1) stated that inhaled corticosteroids were superior to other agents in the treatment of asthma. However, use of inhaled corticosteroids as initial therapy in patients with mild disease and in children was controversial. In the recent guidelines update, (2) the NAEPP Expert Panel examined data comparing chronic use of inhaled corticosteroids and other agents in adults and children with mild or moderate persistent asthma. An overwhelming amount of the data showed that inhaled corticosteroids improve asthma control in children with mild or moderate persistent asthma, as measured by improvements in symptoms and forced expiratory volume in one second (FEV1) and reductions in airway hyperresponsiveness, emergency department visits, hospitalizations, and use of oral corticosteroids. No other medications (i.e., cromolyn [Intal], nedocromil [Tilade], theophylline, leukotriene modifiers) are as effective as inhaled corticosteroids in the long-term control of asthma.
TREATMENT RECOMMENDATIONS
Children. The new asthma treatment recommendations (2) represent a major change from the previous guidelines, which had recommended cromolyn as initial maintenance therapy in children. (1) Inhaled corticosteroids now are recommended in children older than five years with mild persistent asthma (Table 1). (2) According to data from Merck & Co., Inc., the use of leukotriene modifiers also is common, particularly in children (July 2003). However, the updated guidelines (2) state that leukotriene modifiers should not be used as first-line therapy; rather, they are considered second-line or alternative treatment, as are cromolyn, nedocromil, and theophylline. In children five years and younger, the guidelines also recommend inhaled corticosteroids (via dry powder inhaler, nebulizer, or metered-dose inhaler with a face mask) as first-line therapy, although cromolyn and leukotriene modifiers remain alternatives. (2)
Unfortunately, there have been few studies in children younger than five years, and the diagnosis of asthma in infants and children is complicated by the difficulty of obtaining objective measures of lung function. (4) Many children wheeze during the first years of life and do not progress to asthma, (5) and there are no reliable predictors for determining which children will develop asthma. However, physicians who are reluctant to diagnose infants or young children with asthma may be denying these patients life-saving and perhaps disease-modifying medications. To address this problem, the updated guidelines (2) recommend that physicians strongly consider starting long-term therapy for the control of asthma in infants and young children with four or more episodes of wheezing in the past year if the wheezing lasted more than one day and affected sleep and if the patient has risk factors for the development of asthma (i.e., parental history of asthma, atopic dermatitis, allergic rhinitis, or wheezing). (2) Table 2 lists the goals of asthma therapy. (1)
Adults. The treatment recommendations for adults also have changed. The previous guidelines (1) noted that the use of inhaled corticosteroids was preferred in patients with moderate or severe asthma but stopped short of recommending these agents as first-line therapy in patients with mild asthma. In addition to the previously known benefits of inhaled corticosteroid therapy in patients with asthma, recent data (6,7) show that regular use of inhaled corticosteroids can reduce hospital admissions and dramatically decrease deaths from asthma. One study (8) found that compliance with low-dosage inhaled corticosteroid therapy virtually eliminated the risk of death from asthma.
The NAEPP panel (2) reviewed 12 studies of the leukotriene modifiers montelukast and zafirlukast and found that outcome measures "clearly and significantly" favored therapy with inhaled corticosteroids. (8) A recent Cochrane review (9) concluded that leukotriene modifiers have only marginal benefit and should not be recommended as first-line therapy or add-on therapy. Thus, inhaled corticosteroids are recommended as first-line therapy in all patients with persistent asthma. (2)
SAFETY
Although the previous guidelines (1) noted that inhaled corticosteroids are the most effective agents for treating asthma, concerns about adverse effects remained. Studies of older inhaled corticosteroids such as beclomethasone (10) showed a small reduction in children's growth after 12 months of use, but other studies of newer, more potent agents showed no such risk. (11,12)
In the past, physicians may have erred on the side of caution by using the less potent agents in patients with mild disease. However, based on a review of clinical trials that followed children for up to six years, the NAEPP panel (2) found strong evidence that the use of inhaled corticosteroids in recommended dosages does not have long-term, clinically significant, or irreversible adverse effects.
The most significant evidence cited by the NAEPP panel came from the Childhood Asthma Management Program (CAMP) study, (13) which followed more than 1,000 children taking the inhaled corticosteroid budesonide, the mast-cell stabilizer nedocromil, or placebo for an average of six years. Although the CAMP study and other studies reviewed by the panel showed that low to medium dosages of inhaled corticosteroids decreased growth velocity in children (causing a small difference in the rate of growth [approximately 1 cm per year] in the first year of use), this effect was not sustained, and there was no difference in target adult height by the end of the study. A similar study, (14) which included fewer children but followed them for more than 10 years, found similar results. Not surprisingly, children with mild asthma who were taking inhaled corticosteroids had superior outcomes in both studies. (13,14) Thus, the negligible risk of growth reduction is far outweighed by the positive effects of inhaled corticosteroid use in children.
The NAEPP panel (2) also reviewed 16 studies that examined bone mineral density, subcapsular cataracts, glaucoma, and hypothalamic-pituitary-adrenal axis suppression in adults and children treated with corticosteroids; these studies also showed negligible adverse effects from corticosteroid use. A recent study (15) of women 18 to 45 years of age who were taking high dosages of triamcinolone found a potential statistically significant decrease in bone mineral density in the hip (but not the spine) in the older women. However, the decrease was not clinically significant because the rate of loss was very low, and this study has been criticized. (16) Thus, the NAEPP panel (2) concluded that inhaled corticosteroids are safe and recommends them as first-line therapy for children and adults with persistent asthma.
Combination Therapy
The NAEPP panel (2) also considered whether the addition of another long-term asthma-control agent to inhaled corticosteroids would improve outcomes in patients with moderate persistent asthma. The previous guidelines (1) offered several suggestions but did not recommend a specific agent. There is now strong evidence that the addition of a long-acting [beta.sub.2] agonist to a low or medium dosage of an inhaled corticosteroid improves lung function and symptoms and reduces the need for use of a short-acting [beta.sub.2] agonist. (2) Doubling the dosage of the inhaled corticosteroid or adding a leukotriene modifier, theophylline, or cromolyn also improves outcomes, but not as substantially as the combination of an inhaled corticosteroid and a long-acting [beta.sub.2] agonist.
Individual studies (17,18) and a meta-analysis (19) comparing combined long-acting [beta.sub.2] agonists and inhaled corticosteroids with the doubling of the dosage of inhaled corticosteroids have shown that combination therapy reduces asthma exacerbations. The combination of a [beta.sub.2] agonist and an inhaled corticosteroid also has been proved superior to the addition of a leukotriene modifier. (20-22)
The benefits of combination therapy make sense pathophysiologically because of the dual components of asthma: airway inflammation and smooth muscle dysfunction. Inhaled corticosteroids are clearly the most potent anti-inflammatory agents, (1) and long-acting [beta.sub.2] agonists are the most potent bronchodilators. (23) However, long-acting [beta.sub.2] agonists should not be used alone; studies have shown that asthma worsens when these agents are taken without an inhaled corticosteroid. (24,25)
One study (26) published since the updated guidelines were released found that the addition of a long-acting [beta.sub.2] agonist to an inhaled corticosteroid allows physicians to lower the corticosteroid dosage and still maintain asthma control. Thus, combination therapy can be steroid-sparing.
Although the safety of inhaled corticosteroid therapy has been established, physicians should prescribe the lowest dosage possible. In addition, there may be a need to increase the corticosteroid dosage and add a long-acting [beta.sub.2] agonist in patients at high risk for exacerbations, such as those with a history of hospitalizations or emergency department visits because of asthma. (27,28) Combination therapy with an inhaled corticosteroid and a long-acting [beta.sub.2] agonist is more cost effective than treatment with an inhaled corticosteroid plus a leukotriene modifier. (29)
The use of long-acting [beta.sub.2] agonists has been questioned recently because of results from the Salmeterol Multi-center Asthma Research Trial (SMART). (30) The SMART study was designed to assess the safety of the addition of salmeterol to current asthma therapy in patients who had never taken a long-acting [beta.sub.2] agonist. This study of 26,353 patients was stopped after 28 weeks because of concerns in a subset of patients. There were no significant differences between salmeterol and placebo in the primary end point of respiratory-related deaths and life-threatening events requiring interventions (e.g., intubation, ventilation). However, the number of asthma-related deaths was significantly higher in the patients who were taking salmeterol (13 patients) than in those who received placebo (four patients). There was no difference between salmeterol and placebo in the number of deaths among white patients, but eight black patients taking salmeterol died compared with one black patient who received placebo.
Although the findings of the SMART study (30) are of potential concern, it should be noted that the number of asthma-related deaths was significantly lower in the patients taking inhaled corticosteroids (six of 12,254 compared with 11 of 14,099 nonusers), regardless of treatment with salmeterol. Asthma-related deaths also occurred more frequently in patients taking salmeterol who did not use an inhaled corticosteroid. Black patients, who have been shown to have more severe asthma and a higher risk for serious outcomes, (31) were less likely than white patients to use inhaled corticosteroids (38 percent compared with 49 percent). Thus, the SMART study (30) proved that long-acting [beta.sub.2] agonists should not be used without inhaled corticosteroids, that asthma is a serious and life-threatening disease, and that black patients seem to be at increased risk for serious outcomes.
The NAEPP recommendations for moderate persistent asthma have therefore been revised. (2) The preferred treatment for adults and children older than five years is a combination of a long-acting [beta.sub.2] agonist and a low to medium dosage of inhaled corticosteroids. The use of combination therapy in children five years of age and younger is under investigation. However, given the strong evidence from data in older children, the NAEPP guidelines (2) offer two options for treating moderate asthma in this group: the addition of a long-acting [beta.sub.2] agonist to a low dosage of an inhaled corticosteroid, or the use of a medium dosage of an inhaled corticosteroid alone.
Antibiotic Therapy
The NAEPP panel (2) also examined whether patients with acute exacerbations of asthma benefited from antibiotic therapy. Two clinical trials (32,33) found that when antibiotics were prescribed routinely or when suspicion of bacterial infection (e.g., pneumonia, sinusitis) was low, no benefits were associated with antibiotic use. Although viral infections frequently are associated with asthma exacerbations, (34,35) the updated guidelines (2) note that chlamydial, mycoplasmal, and other bacterial infections do not contribute frequently to asthma exacerbations. In fact, data do not support the use of antibiotics in patients with asthma, even when clinical suspicion of bacterial infection is high.
This is part II of a two-part article on asthma treatment recommendations. Part I, "Diagnosis, Monitoring, and Prevention of Disease Progression," appeared in the September 1, 2004, issue of AFP.
See page 1011 for definitions of strength-of-recommendation labels.
An article about how to design systems to improve asthma care in your practice will appear next month in the October 2004 issue of AFP's sister publication, Family Practice Management.
REFERENCES
(1.) National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma: expert panel report 2. Bethesda, Md.: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Heart, Lung, and Blood Institute, 1997; NIH publication no. 97-4051.
(2.) National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma: update on selected topics--2002. Bethesda, Md.: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Heart, Lung, and Blood Institute, 2003; NIH publication no. 02-5074.
(3.) Mintz M. Asthma update: Part I. Diagnosis, monitoring, and prevention of disease progression. Am Fam Physician 2004;70:893-8.
(4.) Kemp JP, Kemp JA. Management of asthma in children [published erratum appears in Am Fam Physician 2002;65:386]. Am Fam Physician 2001;63:1341-8,1353-4.
(5.) Taussig LM, Wright AL, Holberg HJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol 2003;111:661-75.
(6.) Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997;277:887-91.
(7.) Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Lowdose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000;343:332-6.
(8.) Busse W, Raphael GD, Galant S, Kalberg C, Goode-Sellers S, Srebro S, et al. Low-dose fluticasone propionate compared with montelukast for first-line treatment of persistent asthma: a randomized clinical trial. J Allergy Clin Immunol 2001;107:461-8.
(9.) Ducharme F, Hicks G, Kakuma R. Addition of antileukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev 2004;(1): CD003133.
(10.) Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. N Engl J Med 1997;337:1659-65.
(11.) Allen DB, Bronsky EA, LaForce CF, Nathan RA, Tinkelman DG, Vandewalker ML, et al. Growth in asthmatic children treated with fluticasone propionate. Fluticasone Propionate Asthma Study Group. J Pediatr 1998;132(3 pt 1):472-7.
(12.) Shapiro G, Mendelson L, Kraemer MJ, Cruz-Rivera M, Walton-Bowen K, Smith JA. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid-dependent, persistent asthma. J Allergy Clin Immunol 1998;102:789-96.
(13.) Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med 2000;343:1054-63.
(14.) Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000;343:1064-9.
(15.) Israel E, Banerjee TR, Fitzmaurice GM, Kotlov TV, LaHive K, LeBoff MS. Effects of inhaled glucocorticoids on bone density in premenopausal women. N Engl J Med 2001;345:941-7.
(16.) Glazer JL. Bone loss and inhaled glucocorticoids. N Engl J Med 2002;346:533-5.
(17.) Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet 1994;344:219-24.
(18.) Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996;153:1481-8.
(19.) Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA). BMJ 2000;320:1368-73.
(20.) Nelson HS, Busse WW, Kerwin E, Church N, Emmett A, Rickard K, et al. Fluticasone propionate/salmeterol combination provides more effective asthma control than low-dose inhaled corticosteroid plus montelukast [published erratum appears in J Allergy Clin Immunol 2001;107:614]. J Allergy Clin Immunol 2000;106:1088-95.
(21.) Ringdal N, Eliraz A, Pruzinec R, Weber HH, Mulder PG, Akveld M, et al. The salmeterol/fluticasone combination is more effective than fluticasone plus oral montelukast in asthma. Respir Med 2003;97:234-41.
(22.) O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial Am J Respir Crit Care Med 2001;164(8 pt 1):1392-7.
(23.) Johnson M. The preclinical pharmacology of salmeterol: bronchodilator effects. Eur Respir Rev 1991;1:253-6.
(24.) Lemanske RF Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA 2001;285:2594-603.
(25.) Mcivor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. Am J Respir Crit Care Med 1998;158:924-30.
(26.) Busse W, Koenig SM, Oppenheimer J, Sahn SA, Yancey SW, Reilly D, et al. Steroid-sparing effects of fluticasone propionate 100 microg and salmeterol 50 microg administered twice daily in a single product in patients previously controlled with fluticasone propionate 250 microg administered twice daily. J Allergy Clin Immunol 2003;111:57-65.
(27.) Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathological outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. The AMPUL Study Group. Am J Respir Crit Care Med 1999;159(4 pt 1):1043-51.
(28.) Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group [published erratum appears in N Engl J Med 1998;338:139]. N Engl J Med 1997;337:1405-11.
(29.) Stempel DA, O'Donnell JC, Meyer JW. Inhaled corticosteroids plus salmeterol or montelukast: effects on resource utilization and costs. J Allergy Clin Immunol 2002;109:433-9.
(30.) Knobil K, Yancey S, Kral K, Rickard K. Salmeterol Multicenter Asthma Research Trial (SMART): results from an interim analysis [Abstract]. Chest 2003;124:335S.
(31.) Boudreaux ED, Emond SD, Clark S, Camargo CA Jr. Acute asthma among adults presenting to the emergency department: the role of race/ethnicity and socioeconomic status. Chest 2003;124:803-12.
(32.) Shapiro GG, Eggleston PA, Pierson WE, Ray CG, Bierman CW. Double-blind study of the effectiveness of a broad spectrum antibiotic in status asthmaticus. Pediatrics 1974;53:867-72.
(33.) Graham VA, Milton AF, Knowles GK, Davies RJ. Routine antibiotics in hospital management of acute asthma. Lancet 1982;1(8269):418-20.
(34.) Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993;307:982-6.
(35.) Johnston SL, Pattermore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ 1995;310:1225-9.
The author indicates that he does not have any conflict of interest. Dr. Mintz is a member of the advisory board for GlaxoSmithKline and the speaker's bureaus for GlaxoSmithKline, Aventis Pharmaceuticals Inc., and AstraZeneca Pharmaceuticals LP. Sources of funding: none reported.
COPYRIGHT 2004 American Academy of Family Physicians
COPYRIGHT 2004 Gale Group