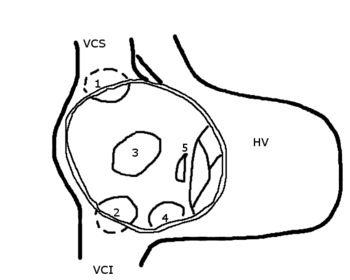
Atrial septal defect (ASD) is one of the most common congenital cardiac anomalies found in the adult population. Although usually asymptomatic in childhood, ASD will be symptomatic in approximately 75 percent of adults. The most common symptoms include fatigue, dyspnea on exertion, and palpitations. However, the presentation of ASD can be protean. We present four patients with secundum ASD with unusual clinical manifestations. Patient 1 had moderately severe mitral regurgitation. Patient 2 had pulmonary edema with generalized left ventricular impairment. Patient 3 had chest pain typical of angina pectoris. Patient 4 had right-to-left shunt following an orthopedic surgical procedure. These patients had chest radiographs and electrocardiograms typical of secundum ASD, but their presentations were uncommon. In three of four of these patients, dramatic resolution of symptoms followed surgical repair of their ASD.
Atrial septal defect (ASD) is one of the most common congenital cardiac anomalies in the adult population. Its prevalence in the western world has been estimated to be between 0.2 and 0.7 per thousand living population.[1] Of the three major types of ASD--ostium secundum, ostium primum, and sinus venosus--ostium secundum defect accounts for nearly 75 percent of lesions.[2]
Due to the absence of symptoms and subtle nature of physical signs, ASD usually is unrecognized for years to decades. However, symptoms develop in 60 percent of patients by age 30 years.[2] The most common presenting symptoms are dyspnea on exertion, fatigue, palpitations due to atrial arrhythmia, and recurrent respiratory illness.[1,3,4] Nevertheless, the presentation of ASD can be protean. Some patients may present atypically or with symptoms typical of other cardiac disease. We present the cases of four patients with ostium secundum ASD with unusual cardiac manifestations.
CASE REPORTS
CASE 1
A 33-year-old black man with a history of rheumatic fever as a child presented with a 2-week history of progressively worsening dyspnea, 9-kg weight gain, ankle edema, and abdominal distention. The patient also complained of one episode of chest pain that occurred 1 week prior to hospital admission. He denied hemoptysis, orthopnea, or paroxysmal noctural dyspnea.
Physical examination revealed mild jugular venous distention, clear lungs, a grade 3/6 holosystolic murmur at the apex, and fixed splitting of the second heart sound. The liver was palpably enlarged and nontender. There was bilateral pitting ankle edema.
An anteroposterior chest radiographs showed marked enlargement of the cardiac silhouette with vascular congestion and alveolar infiltrates consistent with congestive heart failure. Prominent hila secondary to pulmonary arterry congestion were also noted (Fig 1, top). Electrocardiogram showed atrial flutter with 3:1 conduction, right axis deviation, and right bundle branch block (Fig 1, bottom). Echocardiography demonstrated right atrial and right ventricular dilatation and a large secundum ASD. Also noted were moderate mitral regurgitation and symmetric left ventricular hypertrophy. Subsequent cardiac catheterization confirmed the data obtained at echocardiography and a pulmonary-to-systemic flow ratio (Qp/Qs) was calculated to be 3.8:1. The atrial flutter was converted to sinus rhythm with procainamide and direct current cardioversion, and the patient successfully underwent repair of the ASD and mitral valve.
CASE 2
A 55-year-old white woman with a history of hypertension presented with a complaint of recent-onset shortness of breath and bilateral ankle edema. She gave a history of intermittent palpitations but she denied chest pain, orthopnea, or paroxysmal nocturnal dyspnea.
Her physical examination was remarkable for a regular tachycardia with a rate of 110 beats per minute and a grade 2/6 holosystolic murmur at the apex with fixed splitting of the second heart sound. Bibasilar rales were present on auscultation of lung fields. There was also mild bilateral ankle edema.
Anteroposterior chest radiograph demonstrated an enlarged cardiac silhouette with mild pulmonary vascular redistribution. Pleural effusions were noted bilaterally, left greater than right. Electrocardiogram showed sinus tachycardia with first-degree atrioventricular block, premature ventricular complexes, incomplete right bundle branch block, right axis deviation, and left atrial enlargement. Doppler echocardiography revealed a large secundum ASD with large left-to-right shunt, mild mitral regurgitation, moderately severe tricuspid regurgitation, mild pulmonary hypertension, and moderate left ventricular dysfunction. Along with the large ASD noted previously, mild mitral regurgitation, mild pulmonary hypertension (pulmonary artery pressure of 38/16 mm Hg), and severe overall left ventricular impairment were also noted at catheterization. The patient underwent repair of the ASD, and the mitral regurgitation and left ventricular dysfunction resolved post-operatively.
CASE 3
A 41-year-old white man with a medical history of hiatal hernia requiring fundoplication presented with chest pain that radiated down his left arm. He had three such episodes, each associated with dizziness and shortness of breath. There was no exertional component to this pain, nor was it associated temporally with meals. He had no known history of coronary artery disease or history of myocardial infarction.
On presentation, his blood pressure was 140/80 mm Hg, his heart rate was 100 beats per minute, and he was afebrile. Bibasilar rales were found on pulmonary auscultation, as was a regular rhythm with a widely split second heart sound on cardiac auscultation.
Anteroposterior chest radiograph showed mild enlargement of the cardiac silhouette and pleural thickening with fiberoptic lung changes. Electrocardiogram demonstrated normal sinus rhythm with incomplete right bundle branch block, unusual vertical axis for age, and nonspecific ST-T wave changes inferiorly. There was no sign of acute injury or ischemia. Echocardiography uncovered a left-to-right shunt seen on color flow Doppler. Mild right ventricular hypertrophy with right ventricular volume overload was also seen. A left-to-right shunt was also seen at cardiac catheterization but an ASD could not be anatomically localized. The Qp/Qs was calculated to be 1.7:1. Left ventricular function and the coronary arteries were normal. Because cardiac catheterization was unable to locate the suspected ASD, the patient underwent transesophageal echocardiography. This demonstrated a moderately large, fenestrated secundum ASD with normal pulmonary venous drainage and right ventricular volume overload.
With coronary artery disease effectively ruled out as a source of chest pain, gastrointestinal evaluation with esophagogastroduodenoscopy was done followed by empiric treatment with antacids and [H.sub.2]-histaminergic antagonists. No gastrointestinal source of chest pain was found and the patient's symptoms persisted despite treatment.
The patient eventually underwent repair of the ASD, which led to prompt resolution of his chest pain.
CASE 4
A 40-year-old white woman with profound mental retardation following hypoxia at birth, seizure disorder, a history of severely combative behavior, and systolic murmur discovered 3 years prior to admission to the hospital presented with a bimaleolar fracture of her right ankle that occurred while stepping out of bed. Although the patient was unable to speak, her family gave a history of weight gain and dependent edema requiring oral furosemide over the previous 2 months. By parental choice, no formal cardiac evaluation was conducted.
On physical examination, vital signs were stable but the patient was diaphoretic and combative. Her lungs were clear to auscultation and cardiac examination revealed a regular rhythm with a loud pulmonic component of the second heart sound. No murmur was appreciated, and neither edema nor cyanosis was noted.
Anteroposterior chest radiograph revealed prominent pulmonary arteries with pruning. Lung fields were clear (Fig 2, left). Electrocardiogram demonstrated sinus tachycardia with an aberrant premature complex, incomplete right bundle branch block, right ventricular hypertrophy, and abnormal right axis (Fig 2, right).
Under general anesthesia, which included ketamine, succinylcholine, sufentanil, and atracurium, the patient underwent open reduction and internal fixation of the right ankle. During surgery, her arterial oxygen saturation dropped to 40 percent and she became cyanotic. Despite gradually improved oxygenation, she remained desaturated for hours and renal and hepatic function deteriorated markedly.
Echocardiography showed a small left ventricle with abnormal septal motion, a markedly dilated right ventricle with increased wall thickness, and a dilated right atrium. A secundum ASD with right-to-left shunt was also discovered. The patient's postoperative course was further complicated by lethargy, sepsis, and progressive renal and hepatic failure. The parents refused to permit right-sided cardiac catheterization, and the patient died during her hospitalization.
DISCUSSION
Each of these four patients had a different presentation of ASD. One had moderately severe mitral regurgitation, another had pulmonary edema with generalized left ventricular impairment and mild mitral regurgitation, the third had chest pain typical of angina pectoris, and the fourth had right-to-left shunt with hypoxemia following orthopedic surgery. Although each had an electrocardiogram and chest radiograph fairly typical for secundum ASD, their presentations were unique.
Hemodynamically significant mitral regurgitation in the presence of secundum ASD has been described.[3-6] Characteristic abnormality of the mitral valve in patients with ASD consists of thickening and shortening of the chordae tendineae with densely fibrotic valve leaflets.[4,6] Scattered microscopic foci of myxomatous degeneration have also been described.[6] However, there is only a 5 percent incidence of severe mitral regurgitation in the secundum ASD population, with the frequency reaching 15 percent in patients older than 50 years of age. The observation that mitral valve lesions of ASD increase with age has also been attributed to the long-term effects of left ventricular cavity deformity related to the abnormal position and motion of the ventricular septum in response to right ventricular volume overload.[4] Interestingly, abnormal left ventricular geometry is also responsible for the presence of mitral valve prolapse when associated with ASD.[4] The occurrence of significant mitral regurgitation in a 33-year-old patient with ASD without associated mitral valve prolapse is unusual.
Although ASD is usually well tolerated for many years, some adult patients with this condition develop symptoms of left ventricular failure.[1,7,8] Patients with ASD and clinical evidence of congestive heart failure usually have normal overall left ventricular systolic function, with ejection fraction and velocity of circumferential fiber shortening being similar in normal subjects and ASD patients with and without heart failure.[8] These findings would seem to indicate that abnormal left ventricular contractile function is not the cause of heart failure in this patient population. The symptoms can partially be explained by the elevated left ventricular filling pressure observed in some patients with ASD, although the exact mechanism of this elevation is not clear.[1,8] There is some evidence that suggests that diastolic dysfunction may be responsible for some of these findings via the influence exerted by right ventricular filling on left ventricular diastolic function.[4,8] This probably occurs by leftward shift of the interventricular septum caused by right ventricular volume overload.[4,8]
However, patient 2 did have global left ventricular impairment with clinical congestive heart failure. No abnormal interventricular septal motion was noted, nor could this left ventricular dysfunction be attributed to coronary artery disease. Although left ventricular dysfunction has been described in patients with ASD and mitral regurgitation,[3] this patient's mitral regurgitation was not hemodynamically significant. The cause of her left ventricular impairment is not clear. That her left ventricular failure promptly resolved with repair of the ASD confirms their association.
Retrosternal, "angina pectoris-type" chest pain has been described independently of coronary artery disease in patients with ASD.[1,3,9] One study reported that 14 of 84 patients with ASD (16.7 percent) were afflicted with such chest pain; all but one had relief of this symptom following surgical repair of their ASD.[9] Such was the case with our third patient.
Eisenmenger's reaction (pulmonary hypertension with reversal or bidirectional shunt between pulmonary and systemic circulations) occurs in approximately 6 to 13 percent of patients with ASD.[4,10,11] Although pulmonary hypertension as a result of pulmonary vascular disease is the most important complication altering the natural history of ASD, it is not possible to predict which patients will develop severe vascular disease and, hence, shunt reversal.[10] The infrequency of pulmonary vascular disease in patients with ASD can be explained by the deferred development of a significant left-to-right shunt.[4] Before a significant left-to-right shunt is established, pulmonary vascular resistance and pulmonary arterial pressure usually fall to normal levels, thus making the pulmonary vascular bed more tolerant of an appreciable increment in arterial flow.[4] Why some patients are unable to tolerate this increase in pulmonary flow, thereby developing pulmonary vascular disease and eventually right-to-left shunt, is unknown.
Case 4 presented with a previously unrecognized ASD complicated by a right-to-left shunt. This patient did show evidence of chronic left-to-right shunt with dilatation of both the right atrium and right ventricle. As we have no proven diagnosis (permission for autopsy was refused by the parents), we can only conjecture that acute reversal of her shunt could have occurred due to acute increase in pulmonary vascular resistance that may have been due to thromboembolism or even fat embolism that can accompany instrumentation of lower-extremity fractures. She clearly had signs of pulmonary hypertension on an initial cardiac examination preoperatively, but her cyanosis did not develop until midway through her operative procedure.
CONCLUSIONS
These cases illustrate the point that although the uncomplicated ostium secundum ASD is among the most readily diagnosed congenital malformations of the heart in children, this same lesion is often difficult to recognize in the adult. Atrial septal defect may be asymptomatic or it may mimic other more common acquired adult conditions. It is likely to be misdiagnosed as mitral stenosis because of dyspnea, orthopnea, or a delayed pulmonary closure mistaken for an opening snap, a tricuspid flow murmur mistaken for a mitral diastolic murmur and, on chest radiograph, pulmonary plethora due to increased blood flow confused with pulmonary venous congestion.
Mitral regurgitation may be misdiagnosed because the holosystolic murmur of tricuspid regurgitation is prominently heard at the apex, which is occupied by the enlarged right ventricle. Wide splitting of the second heart sound may be correctly recognized but incorrectly attributed to the early aortic valve closure associated with mitral regurgitation rather than the delayed pulmonic closure sound of ASD. An enlarged cardiac silhouette with evidence of pulmonary congestion on chest radiograph in a previously healthy person may be tentatively diagnosed as cardiomyopathy. The chest pain sometimes associated with ASD may be mistakenly diagnosed as coronary artery disease , with diagnosis of ASD being missed if oxygen saturation data from the right side of the heart are not obtained. However, the presence of classic chest radiographic findings of an enlarged cardiac silhouette, dilatation of the main pulmonary artery and its branches with prominent right atrium and right ventricle, coupled with electrocardiogram findings of complete or incomplete right bundle branch block, right axis deviation, and/or right ventricular hypertrophy with occasional atrial arrhythmia should lead the investigator to entertain the diagnosis of secundum ASD.
REFERENCES
[1] Konstantinedes S, Geibel A, Kasper W, Just H. The natural course of atrial septal defects in adults--a still unsettled issue. Klin Wochenschr 1991; 69:506-10
[2] Skokolow M, McIlroy MB. Congenital heart disease (with special reference to adult cardiology): clinical cardiology. Norwalk, Conn: Appleton-Century-Crofts, 1986; 325-33
[3] Fisher J, Platia EV, Weiss JL, Brinker JA. Atrial septal defect in the adult: clinical findings before and after surgery. Cardiovasc Rev Rep 1983; 4:396-410
[4] Perloff JK, Artial septal defect: the clinical recognition of congenital heart disease. Philadelphia: WB Saunders Co, 1987; 272-349
[5] Liberthson RR, Boucher CA, Fallon JT, Buckley MJ. Severe mitral regurgitation: a common occurrence in the aging patient with secundum atrial septal defect. Clin Cardiol 1981; 4:229-32
[6] Feldman T, Borrow KM. Atrial septal defect in adults. Cardiovasc Med 1986; 19-24
[7] Francesco L, Cipriani L, Cocchi A, Zuccala G, Carboni P. Ostium secundum defect in the elderly. J Am Geriatr Soc 1991; 39:60-3
[8] Carabello BA, Gash A, Mayers D, Spann JF. Normal left ventricular systolic function in adults with atrial septal defect and left heart failure. Am J Cardiol 1982; 49:1968-73
[9] Kolbjorn F, Simonsen S, Andersen A, Efksin L. Atrial septal defect of secundum type in the middle aged. Am Heart J 1977; 94:44-54
[10] Cherian G, Uthaman CB, Burairaj M, Sukumar IP, Krishnaswami S, Jairaj PS, et al. Pulmonary hypertension in isolated secundum atrial septal defect: high frequency in young patients. Am Heart J 1983; 105:952-57
[11] Brammel HL, Bogel JHK, Pryor R, Blount SJ. The Eisenmenger syndrome. Am J Cardiol 1971; 28:679-92
COPYRIGHT 1993 American College of Chest Physicians
COPYRIGHT 2004 Gale Group