Platypnea-orthodeoxia is an uncommon syndrome that may occur due to the postpneumonectomy state, cirrhosis of the liver, recurrent pulmonary embolism, and intracardiac shunting. We describe a patient who was found to have a positional change in desaturation after being admitted for dehydration. Workup revealed an atrial septal defect with aneurysm. Following surgical repair, the orthodeoxia resolved. Different mechanisms explain positional desaturation, such as atriovenous malformations at the lung base of cirrhotic patients. In an atrial septal defect, the increased shunting of blood across a malformed septum in an upright position may cause orthodeoxia. This case highlights the necessity of heightened awareness of this syndrome and the need for documenting orthostatic changes in cases of severe hypoxemia.
(CHEST 2000; 118:871-874)
Key words: atrial septal aneurysm; atrial septal defect; hypoxemia; intracardiac shunting; orthodeoxia; platypnea; positional desaturation; zone 1 phenomenon
Abbreviation: TEE = transesophageal echocardiogram
Orthodeoxia is a rare, poorly understood clinical syndrome in which arterial desaturation is accentuated in upright posture and relieved by recumbent position. This is commonly associated with hyperventilation in the upfight position, referred to as platypnea. It is commonly described in patients with chronic lung diseases.
We discuss the case of a patient who exhibited orthodeoxia in the absence of demonstrable lung disease. Extensive workup led to the diagnosis of atrial septal defect with an aneurysm that was surgically repaired, causing the orthodeoxia to cease.
CASE REPORT
A 71-year-old, mentally retarded male patient presented with generalized weakness. He denied having chest pain, shortness of breath, or fever. His medical history was only significant for hypertension. There was no history of industrial inhalation, toxic drug exposure, or tobacco abuse. Physical examination revealed a dehydrated man with poor skin turgor but no evidence of pedal edema, cyanosis, dubbing, or telangiectasia. Orthostatic BP changes were noted. Lung and heart sounds were normal, without audible cardiac murmurs. Laboratory findings were consistent with dehydration.
During hospitalization, nurses observed the patient sitting up in bed talking, then slumping over and becoming cyanotic. Monitors showed no rhythm abnormalities. His heart rate was 80 regular beats/min, and BP was 90/70 mm Hg with spontaneous respiration. When he was placed supine, his condition dramatically improved.
Arterial blood gas measurements revealed an oxygen saturation of 84% and [PO.sub.2] of 50 mm Hg while the patient breathed room air. While breathing 100% oxygen by nonrebreather facemask, oxygen saturation increased to 89% and the [PO.sub.2] to 53 mm Hg. ECG detected a sinus rhythm with right bundle branch block. Chest radiographs demonstrated clear lung fields with a normal cardiovascular silhouette. Bilateral lower extremity venous ultrasounds revealed compressible patent deep veins. A ventilation perfusion lung scan was read as low probability for pulmonary embolism. A high-resolution CT scan revealed the lungs were normal. The diagnosis of pulmonary embolism was further ruled out by a pulmonary angiogram that showed normal pulmonary pressures at 22/10 mm Hg (mean, 14 mm Hg) and good filling of the left and right pulmonary arteries. There was no evidence of filling defects, occlusions, or vascular cutoffs. A transthoracic echocardiogram did not reveal any septal defects and estimated the left ventricular ejection fraction at 60%. It showed a markedly dilated left atrium at 5.5 cm in diameter and a dilated right ventricle 4.6 cm in diameter with preserved contractility.
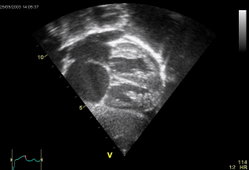
The orthostatic nature of the desaturation was again documented with arterial blood studies while the patient was receiving 100% oxygen by nonrebreather facemask. In supine position, the [PO.sub.2] level was 99 mm Hg and the oxygen saturation rate was 97%; while sitting, the [PO.sub.2] dropped to 38 mm Hg and oxygen saturation to 75%. A transesophageal echocardiogram (TEE) disclosed a probable fenestrated atrial septum with an aneurysm and a bidirectional shunting that was confirmed by cardiac catheterization in supine position.
The 3 x 3-cm defect was surgically repaired. Postoperative TEE did not show any evidence of shunting. The patient experienced an uneventful recovery and cessation of orthodeoxia.
DISCUSSION
Platypnea-orthodeoxia is most commonly recognized in patients with a history of a major pulmonary disorders such as emphysema, recurrent pulmonary embolism, ARDS,[1] pulmonary atriovenous malformations,[2,3] postpneumectomy,[4,5-9] hepatopulmonary syndromes, or cirrhosis of liver.[10,11] It is rarely reported in the absence of overt pulmonary diseases.[4-7,12-14]
Alveolar pressures are uniformly distributed throughout the normal lung. Blood flow depends on the vascular pressure in the different regions of the lung. When in the upright position, blood flow increases from the apex of the lung to the base as gravity causes vascular pressures to be lower at the apex. Patients with chronic lung disease have a parenchymal pulmonary cause of physiologic shunting. In these patients, elevated alveolar pressures alter the ventilatory mechanics by causing pulmonary capillary compression and cessation of blood flow. This has been described as the zone 1 phenomenon.[15] The phenomenon is accentuated in the apical lung portions on assumption of an upright posture with gravitational drop in pulmonary artery pressure. The combination of the two effects could produce a substantial compromise of regional blood flow. These regions are ventilated but underperfused, resulting in respiratory dead space. The increased dead space causes tachypnea and/or dyspnea that further augments air trapping. Alveolar pressures again elevate, creating a repetitive cycle.
Development of platypnea in a postpneumonectomy state should raise the suspicion of the presence of a right to left interatrial shunt.[4,5-9] This may develop because of relative change in the positions of the two atria or mechanical distortion of the fossa ovalis by an upright position. The streaming of the blood flow may be aided by the fact that after pneumonectomy, the interatrial septum would be displaced more to the right, causing the inferior vena cava orifice to become closer to the septal defect.[9] Another probable mechanism to consider is that an increase in the pulmonary vascular resistance after reduction in the pulmonary vascular bed causes an elevation of right ventricular end-diastolic pressures.[11] This, in turn, decreases right ventricular compliance and elevates right atrial pressures. Trepopnea, dyspnea in the lateral position, is more common after pneumonectomy or right middle/lower lobectomy.[16] Constrictive pericarditis in a patient who had undergone pneumonectomy has also been reported as causing orthostatic dyspnea.[17]
Platypnea-orthodeoxia is well documented and better understood in patients with cirrhosis of the liver or hepatopulmonary syndrome.[10,11] When the patient is standing, blood flow follows gravity to the base of the lung, which has true anatomic intrapulmonary vascular dilatations.[3] It is further postulated that these abnormally dilated capillary blood vessels at the lung bases are situated far away from the alveolar epithelium. This decreases the oxygenation of the blood in these segments, which become more intensified in an upright position. A circulating vasodilator that is either produced by or not cleared by the damaged liver may also exist.[11] This vasodilator is thought to be responsible for causing intrapulmonary vascular dilatations.
Many patients with platypnea are found to have normal pulmonary artery pressures.[4] The mechanism of development and marked positional variation in the right to left shunting in such cases is poorly understood. Transient right to left interatrial instantaneous pressure gradient during isovolumic ventricular contraction may cause a small right to left shunt.[18,19] During the phases of the cardiac cycle, the left atrial pressure is generally greater than the right. Early ventricular systole causes a slightly greater right atrial pressure. The interatrial pressure difference is intensified in states that increase the systemic venous return, similar to the release phase of the Valsalva maneuver, or during inspiration. The pooling of pulmonary blood due to decreased intrathoracic pressure in these states accentuates the shunt. "Flow phenomenon" may be another mechanism for platypnea. The preferential flow of blood from the inferior vena cava is directed toward the foramen ovale, as is the case in the prenatal circulatory path.[12,13] Some patients exhibit an abnormally large eustachian valve at the junction of the right atrium and inferior vena cava, which causes selective directional shunting, despite normal atrial pressures.[6,12,13]
Other possible mechanisms to explain shunting in the absence of elevated right heart pressures have been reported. One theory suggests that when an atrial septal defect is present, shunting occurs from the less compliant or stiffer chamber into the more compliant chamber.[4,6] When standing, there is a drop in the right ventricular filling pressures, making the left ventricle relatively more compliant while maintaining right ventricular compliance. In addition, during early diastole, the compliance of the right ventricle is reduced, thus offering greater resistance to blood flow from the right atrium. This leads to enhancement of the shunting across the septal defect without elevation of the right heart pressures. Reduction of intravascular volume can change the right ventricular compliance and cardiac output, thereby increasing the right to left shunting.[2,7]
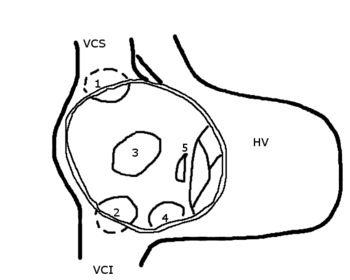
In the case described herein, the patient had an atrial septal defect with an aneurysm. Atrial septal aneurysm formation results from redundancy of the valve of the foramen ovale. The defective septum secundum billows outward to intercept the venous inflow, and the blood is then shunted directly across the opening in the septum. We postulate that due to the dehydrated state, the shunting became even more prominent (Fig 1). Severe kyphosis, aortic root enlargement, and nitrate therapy can also cause the septum secundum to fall away from the primum, thus worsening the shunt.[5]
[Figure 1 ILLUSTRATION OMITTED]
Incidences of platypnea-orthodeoxia have also been described in patients with acute organophosphorous poisoning,so amiodarone-induced lung toxicity,[21] bronchogenic or laryngeal carcinoma,[23,24] cryptogenic fibrosing alveolitis,[25] or autonomic nervous system dysfunction.[26] An elongated ectatic aorta[27] and an aortic aneurysm[28] have also been reported to cause orthodeoxia.
Most cases of platypnea/orthodeoxia described in the literature have been corrected with surgical correction of the shunt, as occurred with our patient. Other treatments for orthodeoxia include prednisone for pericardial effusion following coronary artery bypass graft, and opiate therapy in elderly patients.[14] Treatment with almitrine bismesylate has been shown to potentiate the normal pulmonary hypoxic vasoconstriction, thereby reducing the development of respiratory dead spaces.[29]
CONCLUSION
During the administration of TEE in the supine position, we observed an atrial septal aneurysm coexisting with an atrial septal defect. We suspect upright posture worsened the shunt. Dehydration exacerbated the shunt leading to its discovery.
Greater awareness of orthodeoxia and the necessity of documenting orthostatic changes in saturation levels in all cases of severe hypoxemia are urged. Orthostatic desaturation should prompt further workup and facilitate early recognition of potentially treatable causes. TEE is the ideal technique for septum evaluation and diagnosis of platypnea-orthodeoxia.[30] The presence of an atrial septal defect or a patent foramen ovale should warrant prompt closure to prevent the development of ischemic strokes due to paradoxical embolism.[19]
REFERENCES
[1] Khan F, Parekh A. Reversible platypnea and orthodeoxia following recovery from adult respiratory distress syndrome. Chest 1979; 74:526-528
[2] Seward JB, Hayes DL, Smith HC, et al. Platypnea-orthodeoxia: clinical profile, diagnostic workup, management, and report of seven cases. Mayo Clin Proc 1984; 59:221-231
[3] Robin ED, Laman D, Horn BR, et al. Platypnea related to orthodeoxia caused by true vascular lung shunts. N Engl J Med 1976; 294:941-943
[4] Smeenk FWJM, Postmus PE. Interatrial fight-to-left shunting developing after pulmonary resection in the absence of elevated fight-sided heart pressures: review of the literature. Chest 1993; 103:528-531
[5] Frans E, Timmermans C, Herregods MC, et al. Platypnoea syndrome caused by atrial septal aneurysm. Eur Respir J 1994; 7:2082-2084
[6] Al Khouzaie TA, Busser JR. A rare cause of dyspnea and arterial hypoxemia. Chest 1997; 112:1681-1682
[7] LaBresh KA, Pietro DA, Coates EO, et al. Platypnea syndrome after left pneumonectomy. Chest 1981; 79:605-607
[8] Mercho N, Stoller JK, White RD, et al. Right-to-left interatrial shunt causing platypnea after pneumonectomy: a recent experience and diagnostic value of dynamic magnetic resonance imaging. Chest 1994; 10.5:931-933
[9] Begin R. Platypnea after pneumonectomy. N Engl J Med 1975; 293:342-343
[10] Krowka MJ, Dickson ER, Cortese DA. Hepatopulmonary syndrome: clinical observations and lack of therapeutic response to somatostatin analogue. Chest 1993; 104:515-521
[11] Lange PA, Stoller JK. The hepatopulmonary syndrome. Ann Intern Med 1995; 122:521-529
[12] Sorrentino M, Resnekov L. Patent foramen ovale associated with platypnea and orthodeoxia. Chest 1991; 100:1157-1158
[13] Winters WL Jr, Cortes F, McDonough M, et al. Venoarterial shunting from inferior vena cava to left atrium in atrial septal defects with normal right heart pressures: report of two cases. Am J Cardiol 1967; 19:293-300
[14] Adolph EA, Lacy WO, Hermoni YI, et al. Reversible orthodeoxia and platypnea due to right-to-left intracardiac shunting related to pericardial effusion. Ann Intern Med 1992; 116:138-139
[15] Altman M, Robin ED Platypnea (diffuse zone 1 phenomenon?). N Engl J Med 1969; 281:1347-1348
[16] Alfaifi S, Lapinsky SE. Trepopnea due to interatrial shunt following lung resection. Chest 1998; 113:1726-1727
[17] Mashman WE, Silverman ME. Platypnea related to constrictive pericarditis. Chest 1994; 105:636-637
[18] Nazzal SB, Bansal RC, Fitzmorris SJ, et al. Platypnea-orthodeoxia as a cause of unexplained hypoxemia in an 82-year-old female. Cathet Cardiovasc Diagn 1990; 19:242-245
[19] Strunk BL, Cheitlin MD, Stulbarg MS, et al. Right-to-left interatrial shunting through a patent foramen ovale despite normal intracardiac pressures. Am J Cardiol 1987; 60:413-415
[20] Bouros D, Agouridakis P, Tsatsakis A, et al. Orthodeoxia and platypnoea after acute organophosphorous poisoning reversed by CPAP: a newly described cause and review of the literature. Respir Med 1995; 89:695-628
[21] Papiris S, Maniati MA, Manoussakis MN, et al. Orthodeoxia in amiodarone-induced acute reversible pulmonary damage. Chest 1994; 105:965-966
[22] Fink BW, ed. Congenital heart disease: a deductive approach to its diagnosis. Chicago, IL: Year Book Medical Publishers, 1985; 1
[23] Gacad G, Akhtar N, Cohn JN. Orthostatic hypoxemia in a patient with bronchogenic carcinoma. Arch Intern Med 1974; 134:1113-1115
[24] Schwenk NR, Schapira RM, Byrd JC. Laryngeal carcinoma presenting as platypnea. Chest 1994; 106:1609-1611
[25] Bourke SJ, Munro NC, White JE, et al. Platypnea - orthodeoxia in cryptogenic fibrosing alveolitis. Respir Med 1995; 89:387-389
[26] Fox JL, Brown E, Harrison JK, et al. Platypnea-orthodeoxia and progressive autonomic failure. Am Rev Respir Dis 1989; 140:1802-1804
[27] Popp G, Melek H, Garnett AR Jr. Platypnea-orthodeoxia related to aortic elongation. Chest 1997; 112: 1682-1684
[28] Laybourn KA, Martin ET, Cooper RA, et al. Platypnea and orthodeoxia: shunting associated with an aortic aneurysm. J Thorac Cardiovasc Surg 1997; 113:955-956
[29] Bell RC, Mullins RC 3d, West LG, et al. The effect of almitrine bismesylate on hypoxemia in chronic obstructive pulmonary disease. Ann Intern Med 1986; 105:342-346
[30] Herregods MC, Timmermans C, Frans E, et al. Diagnostic value of transesophageal echocardiography in platypnea. J Am Soc Echocardiogr 1993; 6:624-627
(*) From the Western Reserve Care System (Dr. Acharya), Youngstown, OH; and the Northeastern Ohio Universities College of Medicine (Dr. Kartan), Rootstown, OH.
Manuscript received August 17, 1999; revision accepted March 8, 2000.
Correspondence to: Ritha Kartan, MD, FCCP, Department of Research, Western Reserve Care System, 500 Gypsy Lane, Youngstown, OH 44501
COPYRIGHT 2000 American College of Chest Physicians
COPYRIGHT 2000 Gale Group