Forty years ago, a young British physical chemist predicted a structure for an important class of proteins called coiled coils - including keratin, the basic structural ingredient of skin and hair - based on X-ray diffraction data and some fiddling he did with a broomstick and modeling clay. Until then, scientists thought two spiraling polypeptide strands might twist around one another and join together because a ridge formed by one strand fit into a groove in the second.
In his doctoral thesis, Francis H.C. Crick suggested instead that these helical strands snapped together: Knobs of one fit into holes created by the arrangement of amino acids in its partner, forming a protein with tighter connections than the ridge-in-groove configuration would allow. "It was a very reasonable idea, but it was impossible to check out at the time," recalls Crick, now a Nobel laureate and neuroscientist at the Salk Institute for Biological Studies in San Diego.
Now, researchers have determined that the crystal structure of part of a gene-regulating protein in yeast has exactly the shape Crick proposed. Their work, described in the Oct. 25 SCIENCE, verifies Crick's predictions about the structure of coiled coils, says study director Peter S. Kim, a biochemist with the Howard Hughes Medical Institute at the Whitehead Institute for Biomedical Research in Cambridge, Mass.
The new work also clarifies the atomic nature of so-called leucine zippers stretches of amino acids that bind two proteins into two-molecule complexes called dimers, which regulate gene activity Confirmation that leucine zippers are actually coiled coils will help scientists understand how these proteins regulate the on-and-off switching of genes, comments Steven L. McKnight of the Carnegie Institution of Washington's embryology department in Baltimore.
"But [the discovery] has much broader implications than just gene expression," McKnight told SCIENCE NEWS. It's the first structure of a coiled coil protein, and there are many proteins that use this architecture" - proteins not involved in gene regulation.
The leucine zipper usually consists of a short string of amino acids embedded within a long protein. The sequence on this string repeats so that leucine - a water-repelling (hydrophobic) amino acid - shows up in every seventh slot. The sequence spirals in such a way that all of the leucines face outward on the same side of the spiral, explains McKnight.
When two proteins come together, their hydrophobic amino acids tend to face each other. In 1988, McKnight named this hydrophobic section the leucine zipper because he and his colleagues thought the leucines from two proteins lined up in such a way that they interlocked like teeth in a zipper.
In 1989, Kim's team proposed instead that every third amino acid, and then every fourth one, is hydrophobic - so that amino acids other than leucine are involved.
It's not a zipper at all," Kim says. Parts of one leucine and three other hydrophobic amino acids in one zipper form the sides of a hole" into which leucine from the other zipper fits, he explains.
Coiled coils include cell-surface proteins, muscle fibers and structural proteins in bone and hair, but many are so long that they defy crystallization, says Tom Alber of the University of Utah in Salt Lake City, who supervised the crystallography work in the new study
Erin O'Shea, a graduate student working with Kim, synthesized the 33-amino-acid zipper from the yeast protein. Already, this segment and its crystal structure are helping scientists understand what makes some proteins stay together.
The crystal structure "shows how the amino acid side chains fit in to stabilize the structure," says biophysicist William E Degrado, who designs proteins for Du Pont Merck Pharmaceutical Co. in Wilmington, Del. By making systematic changes in the sequence of amino acids, he can control that stability
In addition, researchers hope to gain new insights into the workings of more complex gene-regulating proteins. Sometimes the leucine zipper helps two different molecules link up, says Kim. He, Alber and others plan to study these zippers further to find out why some molecules link up and others do not.
"I'm very pleased," Crick told SCIENCE NEWS. It's nice to have an idea bear out."
COPYRIGHT 1991 Science Service, Inc.
COPYRIGHT 2004 Gale Group

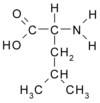
![Leucine Formula (Older Version [Note the diferences in the order of the elements in a molecule]) Leucine Formula (Older Version [Note the diferences in the order of the elements in a molecule])](pics/Leucine_3.png)
