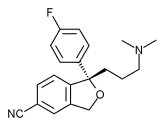
The FDA last month approved Forest Laboratories' Lexapro (escitalopram oxalate), a selective serotonin reuptake inhibitor, for treating generalized anxiety disorder.
GAD, one of the most common mental illnesses in the United States, affects some 4 million Americans annually, Forest reported.
In a separate release, Forest announced the results of a clinical study showing that Lexapro is as effective and well tolerated as the most-prescribed SSRI, Pfizer's Zoloft (sertraline hydrochloride).
Zoloft, which is not approved for GAD, had $2.8 billion in sales for the 12 months ended September 2003, according to IMS Health. Comparatively, Lexapro's sales were placed at $244.7 million for fiscal, year 2003 ended March 31, Forest reported. Forest s SSRI has been on the market since August 2002.
Although Pfizer is not planning to seek a GAD indication for Zoloft, a Pfizer study introduced to the Anxiety Association Disorders of America last year established its efficacy in GAD patients. And Pfizer filed a new drug application for Pregabalin, a Neurontin-like drug, in October that is seeking a GAD-treatment indication.
COPYRIGHT 2004 Reproduced with permission of the copyright holder. Further reproduction or distribution is prohibited without permission.
COPYRIGHT 2004 Gale Group