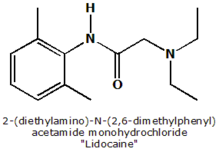
Objective: Topical anesthesia for flexible bronchoscopy can be administered via transcricoid injection, nebulizer, or directly through the bronchoscope in a "spray as you go" fashion. We performed a prospective, randomized, double-blind, placebo-controlled trial to evaluate whether nebulized lidocaine provides additional benefit and reduces the total anesthetic dose required during bronchoscopy.
Setting: Tertiary care university hospital.
Methods: One hundred fifty patients (93 men; age, 20 to 89 years) undergoing diagnostic flexible bronchoscopy were randomized to receive either 4 mL of 4% lidocaine (160 mg) or 4 mL of saline solution as placebo via nebulization. Combined sedation was achieved using 5 mg of IV hydrocodone and midazolam boluses. Supplemental lidocaine doses and total midazolam required as judged by the bronchoscopist were recorded for each patient. After the procedure, both bronchoscopists and patients charted their perception of cough on a 10-cm visual analog scale (VAS). Similarly, patients recorded their discomfort related to the procedure on a 10-em VAS.
Results: The most common procedures were BAL in 77 cases (51%), transbronchial biopsy in 40 cases (27%), and transbronchial needle aspiration in 34 cases (23%). Outcome parameters, including hemodynamic findings, duration of the procedure, cough scores for physicians and patients, discomfort score for patients, midazolam doses, and supplemental lidocaine doses, were similar in both groups. Mean total lidocaine dose required in the lidocaine group was 318 [+ or -] 41 mg and was significantly higher than the total dose required in the placebo group (157 [+ or -] 44 mg [[+ or -] SD]) [p < 0.001].
Conclusion: Additional nebulized lidocaine cannot be recommended for flexible bronchoscopy performed under combined sedation. (CHEST 2005; 128:1756-1760)
Key words: bronchoscopy; lidocaine; midazolam; nebulization; sedation
Abbreviation: VAS = visual analog scale
**********
Despite having been introduced > 30 years ago, bronchoscopic practice is still not standardized. (1) Topical anesthesia for flexible bronchoscopy can be achieved in several ways, including administration of anesthetic agents by tracheal injection, via nebulizer, or directly through the bronchoscope in a "spray as you go" fashion. (2,3) Cocaine, benzocaine, tetracaine, and lidocaine seem to be equally effective, but lidocaine has the better safety profile and is therefore most commonly used. (4,5) Although usually safe, lidocaine can cause arrhythmias, seizures, cardiorespiratory arrest and, rarely, death. (6-8) Side effects become more likely if lidocaine plasma levels are > 5 mg/L, particularly in patients with decreased liver function. (9) Lidocaine instilled endoscopically rapidly enters the circulation, and a correlation has been reported between plasma levels and the total dose administered during bronchoscopy. (10) Alternatively, nebulization has been shown to produce equivalent anesthetic effects compared to endobronchial administration while resulting in significantly lower lidocaine plasma levels. (3,11,12) Furthermore, patient preference seems to favor the nebulized route. (3,13) Foster and Hurewitz (14) demonstrated that nebulized lidocaine can reduce the requirement for supplemental topical anesthesia administered via injection through the bronchoscope. Similarly, Gjonaj et al (15) reported that 50% of the patients receiving aerosolized lidocaine did not require supplemental lidocaine. However, all these studies had a small sample size and were performed without combined sedation, despite the widespread use of the combination of a benzodiazepine and an opiate. (1,2,16,17) Recently, this combination has been shown to improve operating conditions due to its antitussive effects and to improve patient satisfaction. (17) Thus, combined sedation has been suggested to be adopted as standard sedation for patients without contraindications. (17) We therefore performed a prospective, randomized, placebo-controlled, double-blind trial to evaluate whether nebulized lidocaine provides additional benefit and reduces the total anesthetic dose required during bronchoscopy in patients receiving combined sedation with midazolam and hydrocodone.
MATERIALS AND METHODS
Patients
The study has been approved by the institutional ethical committee. After obtaining written informed consent, 150 consecutive patients undergoing diagnostic flexible bronchoscopy were prospectively randomized utilizing a computer system to receive either nebulized 4% lidocaine or placebo in a double-blind fashion. Intubated patients, those receiving propofol (eg, IV drug abuse), and patients requiring endobronchial ultrasound examination were not included in this trial.
Study Design
Bronchoscopies were performed transnasally with the patients in the semirecumbent position. Pulse oximetry was recorded continuously during the procedure, and automated noninvasive BP was monitored every 5 min. Supplemental oxygen was offered at 4 L/min via nasal cannula to all patients. In case of desaturation < 90%, oxygen delivery was increased to 6 L/min. (18)
After randomization, patients had received 4 mL of 4% lidocaine (160 mg of lidocaine) or 4 mL of saline solution as placebo delivered by nebulization (MPV Truma; Munich, Germany) over 15 min immediately before bronchoscopy. Nasal anesthesia was achieved by spraying 10% lidocaine in the nasopharynx (four times) and oropharynx (two times). Lidocaine administered through the bronchoscope was defined as supplemental lidocaine. Bronchoscopists were advised to instill 3-mL aliquots of 1% lidocaine over the vocal cords, onto the trachea and both right and left main bronchi. All doses of supplemental local anesthesia required as judged by the bronchoscopist were recorded for each patient.
All patients received 5 mg of IV hydrocodone immediately before flexible bronchoscopy. (17) Thereafter, conscious sedation was achieved initially with 2 mg of IV midazolam followed by further 1- to 2-mg intermittent boluses during the procedure at the discretion of the endoscopist. (17) The total doses of midazolam were documented. Diagnostic bronchoscopic procedures were performed depending on the clinical setting. At the end of the procedure, bronchoscopists charted their perception of cough during the procedure on a 10-cm visual analog scale (VAS). A higher score indicated greater frequency of cough. Two to 3 h after bronchoscopy, patients were asked to record both their perception of cough and discomfort related to the procedure on a 10-cm VAS, where 0 = no discomfort or cough, and 10 = greater levels of discomfort or incessant cough. BP and heart rate were also measured.
Data Analysis
Analysis was performed using software (Statistical Package for Social Sciences, version 11.5 for Windows; SSPS; Chicago, IL). The [chi square] test was used to compare the distribution of diagnostic procedures in the lidocaine and placebo groups. The Mann-Whitney U test was used to calculate the level of significance between two means values in the two groups. Power and sample sizes were calculated using the supplemental lidocaine dose as the primary outcome variable. (14) Considering 40 mg of supplemental lidocaine as a significant difference between the groups, 75 patients would be needed in each study arm to achieve a significance level of < 0.05 with a power of 0.9. Results are given as mean [+ or -] SD unless otherwise stated. The VAS data are presented as median (range).
RESULTS
Patient characteristics are presented in Table 1. All examinations could be completed as planned. There were no significant differences in age, gender, and indication for bronchoscopy between both groups. Distribution of different invasive bronchoscopic procedures performed were also similar in both groups (Table 2). The most common procedures were BAL in 77 cases (51%), followed by bronchial washings in 63 cases (42%), transbronchial biopsy in 40 cases (27%), and transbronchial needle aspiration in 34 cases (23%) cases, respectively. In 88 cases (59%), BAL or transbronchial biopsy was performed, either alone or in combination.
Hemodynamic findings before, during, and after bronchoscopy for both groups are illustrated in Table 3. Oxygen requirement was 4.5 [+ or -] 1.5 L/min and 4.7 [+ or -] 0.5 L/min in the lidocaine and placebo groups, respectively (p = 0.139) [[+ or -] SD]. The lowest oxygen saturation during the procedure was 93.9 [+ or -] 1.8% and 94.6 [+ or -] 4.7% in the lidocaine and placebo groups, respectively (p = 0.458). Three cases of oxygen desaturations < 90% were noted in the lidocaine group as compared to six cases in the placebo group (p = 0.319).
Cough scores for physicians and patients as well as the discomfort score for patients are shown in Table 4. There was no statistical significance between both groups in regard to cough score evaluated by the physician (p = 0.062), by the patient himself (p = 0.225), or discomfort of the patient (p = 0.188), respectively.
Mean dose of supplemental lidocaine was 158 [+ or -] 41 mg in the lidocaine group, as compared to 157 [+ or -] 44 mg in the placebo group (p = 0.157). Accordingly, mean total lidocaine dose required in the lidocaine group was 318 [+ or -] 41 mg and was significantly higher than the total dose required in the placebo group (157 [+ or -] 44 mg) [p < 0.001]. Midazolam doses were similar in both groups (p = 0.237).
DISCUSSION
This study demonstrates no additional benefit of nebulized lidocaine in reducing the total dose of topical anesthetic administered for flexible bronchoscopy in patients receiving combined sedation with midazolam and hydrocodone. Indeed, patients treated with nebulized lidocaine received overall greater amounts of lidocaine than the placebo group. Furthermore, the administration of aerosolized lidocaine prior to bronchoscopy did not significantly improve patient comfort or prevent cough. Operating conditions, inferred from the duration of the procedure and the amount of midazolam required, were not improved either. Considering that currently 95% of the examinations are carried out under sedation, (2,16,19) these results suggest that nebulized lidocaine has no role as local anesthesia for flexible bronchoscopy, perhaps due to the additional effect of hydrocodone on cough suppression.
A PubMed search did not reveal any previous studies examining the use of nebulized lidocaine in patients receiving combined sedation with midazolain and hydrocodone. Former studies (14,15,20) were performed utilizing either lidocaine alone or a combination with a benzodiazepine. In one of these studies, Gove et al (21) reported a reduction in the duration of the procedure with nebulized lidocaine, both alone and combined with IV diazepam. However, midazolam has replaced diazepam in most centers due to its shorter duration of action compared to diazepam and is now by far the most common sedative used during bronchoscopy. (2,16) To our knowledge, this is the first study assessing the value of additional nebulized lidocaine in patients receiving combined sedation with midazolam and hydrocodone.
Isaac et al (13) compared three different methods of local anesthesia, including nebulization, and transcricoidal and bronchoscopic injection. The transcricoid method produced better working conditions than nebulization, although both techniques were satisfactory. In another study (20) with a similar design, all patients in the nebulized group required supplemental local anesthesia, compared to 1 of 18 patients in the transcricoid injection group. Webb et al (22) compared the administration of lidocaine using the transcricoid method with the "spray as you go" technique. The required supplemental lidocaine dose was higher in the transcricoid injection group compared to the "spray as you go" group. (22) Although deemed effective, transcricoid administration of lidocaine has not been widely used. (1) The main reason seems to be that almost one third of the patients receiving a transcricoid injection find this procedure unpleasant. (22) Moreover, transcricoid puncture may be unacceptable in some patients, specially if coagulation abnormalities are presented. (13) Foster and Hurewitz (14) demonstrated a reduction of supplemental lidocaine doses required for flexible bronchoscopy if nebulized lidocaine was previously administered. Despite the fact that the results were statistically significant, it is difficult to judge the clinical relevance of this finding, particularly due to the small number of patients in each group (n = 5 and n = 9, respectively).
One possible limitation of this study is that tolerability may have been overestimated 2 h after the procedure because of the amnesic effect of midazolain. According to several previous studies, (23-25) wake-up time for combined sedation with an opiate and benzodiazepine is 35 to 60 min and discharge time is 75 to 120 min after the procedure. We therefore believe that it is fair to assume that patients were able to estimate their discomfort during flexible bronchoscopy 2 h after the procedure.
Although the total amount of lidocaine required in our study was significantly higher in the nebulized lidocaine group compared to the placebo group, the total lidocaine doses were far below the recommended maximum dose by the British Thoracic Society guidelines (8.2 mg/kg body weight, or approximately 580 mg for a 70-kg patient). (2) In addition, we did not observe any adverse effect due to lidocaine administration. While all monitored outcomes including patient comfort were similar in both groups, it seems rational to limit lidocaine dosage to the minimum amount required, thus ensuring patient safety. Therefore, additional nebulized lidocaine cannot be recommended for flexible bronchoscopy performed under combined sedation.
ACKNOWLEDGMENT: The authors thank the endoscopy staff (Esther Gysin, Dusan Jovic, Bjorn Fehrke, Monika Kohler, Sylvie Groelly, Michael Ortmann, Beatrice Lehner, Brigitte Koch, Catherine Keller, Margot Brenneisen) for their excellent collaboration.
REFERENCES
(1) Smyth CM, Stead RJ. Survey of flexible fibreoptic bronchoscopy in the United Kingdom. Eur Respir J 2002; 19:458-463
(2) British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001; 56(suppl):i1-i21
(3) Keane D, McNicholas WT. Comparison of nebulized and sprayed topical anaesthesia for fibreoptic bronchoscopy. Eur Respir J 1992; 5:1123-1125
(4) Sanderson DR. Lignocaine for topical anesthesia in fiberoptic bronchoscopy. Respiration 2000; 67:9-10
(5) Teale C, Gomes PJ, Muers MF, et al. Local anaesthesia for fibreoptic bronchoscopy: comparison between intratracheal cocaine and lignocaine. Respir Med 1990; 84:407-408
(6) Day RO, Chalmers DR, Williams KM, et al. The death of a healthy volunteer in a human research project: implications for Australian clinical research. Med J Aust 1998; 168:449-451
(7) Wu FL, Razzaghi A, Souney PF. Seizure after lidocaine for bronchoscopy: case report and review of the use of lidocaine in airway anesthesia. Pharmacotherapy 1993; 13:72-78
(8) Ritchie JM, Greene NM. Local anesthetics. In: Hardman JG, Limbird LE, and Gilman AG, eds. Goodman and Gilman's the pharmacological basis of therapeutics. 8th ed. New York, NY: Pergamon, 2000; 320-322
(9) Sutherland AD, Santamaria JD, Nana A. Patient comfort and plasma lignocaine concentrations during fibreoptic bronchoscopy. Anaesth Intensive Care 1985; 13:370-374
(10) Milman N, Laub M, Munch EP, et al. Serum concentrations of lignocaine and its metabolite monoethylglycinexylidide during fibre-optic bronchoscopy in local anaesthesia. Respir Med 1998; 92:40-43
(11) Eyres RL, Bishop W, Oppenheim RC, et al. Plasma lignocaine concentrations following topical laryngeal application. Anaesth Intensive Care 1983; 11:23-26
(12) Korttila K, Tarkkanen J, Tarkkanen L. Comparison of laryngotracheal and ultrasonic nebulizer administration of lidocaine in local anaesthesia for bronchoscopy. Acta Anaesthesiol Scand 1981; 25:161-165
(13) Isaac PA, Barry JE, Vaughan RS, et al. A jet nebuliser for delivery of topical anesthesia to the respiratory tract: a comparison with cricothyroid puncture and direct spraying for fibreoptic bronchoscopy. Anaesthesia 1990; 45:46-48
(14) Foster WM, Hurewitz AN. Aerosolized lidocaine reduces dose of topical anesthetic for bronchoscopy. Am Rev Respir Dis 1992; 146:520-522
(15) Gjonaj ST, Lowenthal DB, Dozor AJ. Nebulized lidocaine administered to infants and children undergoing flexible bronchoscopy. Chest 1997; 112:1665-1669
(16) Pickles J, Jeffrey M, Datta A, et al. Is preparation for bronchoscopy optimal? Eur Respir J 2003; 22:203-206
(17) Stolz D, Chhajed PN, Leuppi JD, et al. Cough suppression during flexible bronchoscopy using combined sedation with midazolam and hydrocodone: a randomised, double blind, placebo controlled trial. Thorax 2004; 59:773-776
(18) Chhajed PN, Glanville AR. Management of hypoxemia during flexible bronchoscopy. Clin Chest Med 2003; 24:511-516
(19) Colt H, Prakash U, Offord K. Bronchoscopy in North America. J Bronchol 2000; 7:8-25
(20) Graham DR, Hay JG, Clague J, et al. Comparison of three different methods used to achieve local anesthesia for fiberoptic bronchoscopy. Chest 1992; 102:704-707
(21) Gove RI, Wiggins J, Stableforth DE. A study of the use of ultrasonically nebulized lignocaine for local anaesthesia during fibreoptic bronchoscopy. Br J Dis Chest 1985; 79:49-59
(22) Webb AR, Fernando SS, Dalton HR, et al. Local anaesthesia for fibreoptic bronchoscopy: transcricoid injection or the 'spray as you go' technique? Thorax 1990; 45:474-477
(23) Greig JH, Cooper SM, Kasimbazi HJ, et al. Sedation for fibreoptic bronchoscopy. Respir Med 1995; 89:53-56
(24) Webb AB, Doherty JF, Chester MR, et al. Sedation for fibreoptic bronchoscopy: comparison of alfentanil with papaveretum and diazepam. Respir Mead 1989; 83:213-217
(25) Bright E, Roseveare C, Dalgleish D, et al. Patient-controlled sedation for colonoscopy: a randomized trial comparing patient-controlled administration of propofol and alfentanil with physician-administered midazolam and pethidine. Endoscopy 2003; 35:683-687
* From the Division of Respiratory Medicine and Pulmonary Cell Research, University Hospital Basel, Basel, Switzerland.
Manuscript received December 15, 2004; revision accepted March 14, 2005.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal. org/misc/reprints.shtml).
Correspondence to: Daiana Stolz, MD, Division of Respiratory Medicine and Pulmonary Cell Research, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; e-mail: daistolz@yahoo.com
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group