METHOD OF PREPARATION
Note: Calibrate the mold to be used with this base prior to preparing the tablet triturates for the first time. Also, the ratio of lactose:sucrose and ethanol:water can be varied for the desired hardness and release of the sublingual tablets.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Mix the lorazepam, lactose, and sucrose until uniform.
4. Moisten the powders with a 1:1 mixture of the alcohol: water mixture until a dough consistency is obtained.
5. Separate the mold and place the cavity plate on a hard surface, such as a pill tile.
6. Press the drug mixture into the cavities until uniform and firm.
7. Place the cavity plate over the peg plate of the mold and gently press the cavity plate down over the pegs, leaving the formed tablets on top of the pegs.
8. Allow the tablets to air dry.
9. Remove the tablets carefully.
10. Package and label.
PACKAGING
Package in a well-closed, light-resistant container.
LABELING
Use only as directed. Keep out of reach of children.
STABILITY
These tablets should be stable for up to 6 months.1
USE
Lorazepam su lingual tablets are used in the treatment of anxiety disorders or for the short-term relief of symptoms of anxiety or anxiety associated with depressive symptoms.2
QUALITY CONTROL
Quality-control assessment can include average tablet weigh, actual yield compared to theoretical yield, and physical observation.
DISCUSSION
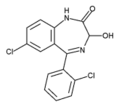
Lorazepam (C^sub 15^H^sub 10^C^sub 12^, N^sub 2^O^sub 2^, MW 321.16) occurs as a white or practically white, practically odorless powder. It is insoluble in water and sparingly soluble in alcohol. It should be stored in tight, light-resistant containers. It melts at about 173 deg C, and has pK^sub a^ values of 1.3 and 11.5, and an octanol/pH 7.4 buffer partition coefficient of 2.4.
Lactose, anhydrous (C^sub 12^H^sub 22^O^sub11^, MW 342.30); lactose monohydrate (C^sub 12^H^sub 22^O^sub 11^ * H^sub 2^O, MW 360.31); (milk sugar, saccharum lactis) is available either anhydrous or as the monohydrate. Commercially, it is available as anhydrous alpha-lactose, alpha-lactose monohydrate, and anhydrous beta-lactose; it is available in different grades and different particle characteristics. It is widely used as a diluent in numerous dosage forms, and in lyophilized products and infant formulas. It is soluble in water to the extent of about 1 g in 4.63 mL but is practically insoluble in ethanol.3
Sucrose (beet sugar, cane sugar, refined sugar, saccharose) is obtained from sugar cane, sugar beet, or other sources. It contains no added substances. It occurs as colorless crystals, crystalline masses or blocks, or as a white, crystalline powder that is odorless and has a sweet taste. It has a melting range of 160 deg C to 186 deg C with some decomposition. Sucrose is soluble in water (1 g in 0.5 mL), alcohol (1 g in 400 mL), and 95% ethanol (1 g in 170 mL), and is practically insoluble in chloroform. Sucrose is stable at room temperature.4
Alcohol (ethyl alcohol, ethanol, grain alcohol) is a clear, colorless mobile and volatile liquid with a slight, characteristic odor and a burning taste. Alcohol USP refers to 95% ethanol, and dehydrated alcohol refers to 99.5 % alcohol. The specific gravity of Alcohol USP is between 0.812 and 0.816 and its boiling point is 78.15 deg C. It is miscible with chloroform, glycerin, and water and its solutions may be sterilized by autoclaving or by filtration. It should be stored in a cool place.5
Purified water is water that is obtained by distillation, ion exchange, reverse osmosis, or some other suitable process. Water has a specific gravity of 0.9971 at room temperature, a melting point at O deg C, and a boiling point at 100 deg C. It is miscible with most polar solvents and is chemically stable in all physical states (ice, liquid, and steam).6
References
1. United States Pharmacopeial Convention, Inc. United States Pharmacopeia 25/National Formulary 20. Rockville, MD:US Pharmacopeial Convention, Inc; 2002:1021, 2053-2057, 2383.
2. McEvoy GK. AHFS Drug Information-2002. Bethesda, MD:American Society of Health-System Pharmacists; 2002:2394-2396.
3. Kibbe AH. Lactose. In: Kibbe A, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:276-285.
4. Hamlow EE, Armstrong NA, Pickard A. Sucrose. In: Kibbe A, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:539-543.
5. Weller PJ. Alcohol. In: Kibbe A, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:7-9.
6. Ellison A, Nash RA, Wilkin MJ. Water. In: Kibbe A, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:580-584.
Copyright International Journal of Pharmaceutical Compounding Mar/Apr 2003
Provided by ProQuest Information and Learning Company. All rights Reserved