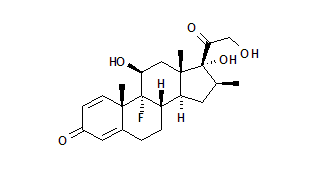
The Food and Drug Adminsitration has granted marketing clearance to Luxiq (betamethasone valerate) Foam, 0.12%, a novel, fast-acting foam formulation for relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses of the scalp. Connetics Corp. said the foam will be available by prescription in approximately six weeks. The approval was based on the results of a multi-center, double-blind active and placebo-controlled Phase III clinical trial of 190 patients, 172 of whom completed the study as directed and were evaluable for efficacy. The data demonstrated that patients treated with Luxiq showed significant improvements for all primary endpoints, including erythema, plaque thickness and scaling. In addition, global assessments performed by the investigators showed that 72 percent of patients treated with Luxiq had complete or almost complete clearance of disease compared with 47 percent treated with a currently approved betamethasone valerate solution and 21 percent treated with placebo.
COPYRIGHT 1999 Lebhar-Friedman, Inc.
COPYRIGHT 2000 Gale Group