Sexually transmitted infections are an important cause of morbidity and mortality throughout the world. Over 300 000 cases of sexually transmitted infection were diagnosed in genitourinary medicine clinics in England in 1996.[1] Among these infections, the commonest were genital warts (over 50 000 first attacks), non-specific genital infection (over 50 000 cases), proved chlamydial infection (over 30 000 cases), genital herpes (about 15000 first attacks), and gonorrhoea (over 10 000 cases).
Methods
In selecting material for this article, I used the current publications section of the journal Sexually Transmitted Infections (formerly Genitourinary Medicine) and searched Medline under the following headings: Chlamydia trachomatis, human papillomavirus, genital herpes, famciclovir, and valaciclovir.
Epidemiological changes
Rates of infection with gonorrhoea in England had been declining rapidly, even before the government launched its Health of the Nation initiative in 1992.[2] Unfortunately, soon after the target (a fall in gonorrhoea rates of at least 20% by 1995) had been reached, the trend was reversed: diagnoses of gonorrhoea in genitourinary medicine clinics rose by 20% between 1995 and 1996.[1] Over the same period, diagnoses of genital infection with C trachomatis rose by 11% and those of genital warts by 5%.[1]. Among homosexual men, there was a steady increase in the incidence of genital warts between 1990 and 1995, while there was no clear trend in the incidence of gonorrhoea (fig 1).
[Figure 1 ILLUSTRATION OMITTED]
Increasing rates of antibiotic resistant strains of gonorrhoea, such as penicillinase producing Neisseria gonorrhoeae (PPNG) and tetracycline resistant N gonorrhoeae (TRNG) (fig 2) have necessitated a switch to the use of fluoroquinolones in some areas. Reports from the United Kingdom and other countries of an increasing number of isolates of gonococcus that are resistant to fluoroquinolones, such as ciprofloxacin, are of particular concern.[4] These reports come at a time when quinolones have overtaken penicillin as the drug of first choice for gonorrhoea in genitourinary medicine clinics in the United Kingdom.[5] Reduced sensitivity to cephalosporins has also been reported and this will doubtless cause cephalosporin resistant gonococci to spread in the future.[7]
[Figure 2 ILLUSTRATION OMITTED]
Much of the gonorrhoea that is resistant to fluoroquinolones is spreading from South East Asia,[6] but some is coming from Russia.7 The former Soviet Union is also an important source of syphilis, which has shown a rapid and substantial increase in incidence there during the 1990s.[8] An epidemic of syphilis in eastern Poland has also been noted, and is said to be spreading rapidly, together with HIV, into neighbouring countries.[9] A severe outbreak of syphilis among heterosexuals in Bristol seemed to originate within the United Kingdom.[10] At the time of the published report, 27 cases had been found,[10] but further cases have since been discovered. The results of a three year survey of syphilis in pregnancy and congenital syphilis by the Communicable Disease Surveillance Centre, the British Cooperative Clinical Group (Genitourinary Medicine), and the British Paediatric Surveillance Unit should be published soon. These will confirm the need for continued vigilance.
Diagnostic methods
Amplification assays
C trachomatis infection is often asymptomatic and may cause long term morbidity, especially in women.[11] Up to a third of inadequately treated women may go on to develop pelvic inflammatory disease, and a fifth of these women may become infertile and a 10th may have ectopic pregnancy.[11] In addition, infertility often arises from undiagnosed asymptomatic chlamydial infection.
For years, cell culture has been considered the ideal method of diagnosing C trachomatis, but the less sensitive enzyme immunoassays have been widely used in laboratories with a high work load. Recently, nucleic acid amplification techniques (ligase chain reaction and polymerase chain reaction) have been evaluated for the detection of chlamydia.[12] The most recent development is transcription mediated amplification, which amplifies ribosomal RNA.
Ligase chain reaction assay has also been developed to detect N gonorrhoeae and chlamydia from the same specimen.[13] Not only does the sensitivity of ligase chain reaction far exceed that of older methods for chlamydia detection, it also has 100%--or near 100%--specificity.[13] The sensitivity falls when first void urine samples are used instead of genital swabs. Nevertheless, the sensitivity for C trachomatis in urine still far exceeds that achieved by culturing swabs.[13] Alternatively, the use of amplified enzyme immunoassay on urine has been advocated.[11]
Antibody tests for herpes simplex types
Antibodies to herpes simplex virus type 2 have been found in 7.6% of blood donors and in 22.7% of people attending the genitourinary medicine clinic at a London hospital.[14] However, in only 32% of those attending the clinic who had antibodies to herpes simplex virus type 2 was a clinical diagnosis of genital herpes made.[14] Most genital herpes is transmitted by people who are unaware of their diagnosis, and this gives rise to a huge problem in minimising transmission. However, recent advances have enabled asymptomatic carriers to be identified. Accurate serological methods of detecting the type of herpes simplex virus are becoming available in both the United States and Europe.[15] Unfortunately, some of the serological tests that are already available are very inaccurate--for example, some kits gave the correct diagnosis in only 33%-55% of patients tested.[15] Furthermore, no test can distinguish between oral and genital herpes simplex virus type 1 infection. This is important because in some areas herpes simplex virus type 1 is now more often the cause of newly diagnosed genital herpes than is herpes simplex virus type 2.[16-18] A recent survey showed that people attending genitourinary medicine clinics want to know their herpes simplex virus serostatus.[19] Some doctors, however, may prefer to use these tests selectively rather than for screening all those attending the clinic.[20]
New drugs
Drugs against HIV
The currently available antiretroviral drugs act to inhibit either the reverse transcriptase enzyme or the protease enzyme. The first three protease inhibitors were licensed in 1996. Their place in clinical practice is now well established,[21] and a fourth, nelfinavir, has also now been licensed.
Triple therapy, consisting of a protease inhibitor plus two nucleoside analogue reverse transcriptase inhibitors, has been shown to be better than dual therapy with two nucleoside analogue reverse transcriptase inhibitors.[21] The proportion of patients whose disease progressed to AIDS or death fell from 11% to 6% with the addition of the protease inhibitor indinavir.[22]
A new class of antiretroviral drug has become available recently--the non-nucleoside reverse transcriptase inhibitors, such as nevirapine. This brings the total number of antiretroviral drugs available in the United Kingdom (either licensed or on a named patient basis) to 13.
Drugs against herpes simplex
Aciclovir, a nucleoside analogue, has been available for over 15 years and has an admirable safety record. However, it is awkward to take because tablets are required five times a day for first episodes and, when necessary, for troublesome recurrences. For those who have six or more recurrences a year, continuous suppression, most often using aciclovir 400 mg twice daily, is successful in preventing most--and sometimes all--of these.
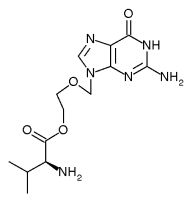
Two new nucleoside analogue prodrugs, valaciclovir and famciclovir, have come into use recently, and more has been learnt about how best to prescribe them. Valaciclovir is almost completely converted to aciclovir in the body and is therefore expected to match aciclovir's safety record. Valaciclovir at a dose of 500 mg twice daily is equivalent in efficacy to aciclovir at a dose of 200 mg five times a day as episodic treatment of recurrences.[23] Valaciclovir can also be used to suppress recurrent genital herpes; and here it has the advantage of requiring a single daily dose of 500 mg.[24] However, unlike the other two agents, valaciclovir is not yet licensed for this purpose in the United Kingdom.
Famciclovir is different from the other compounds in that it is converted to penciclovir, and evidence from studies in mice show that famciclovir and valaciclovir have different effects on the course of herpes.[25] When valaciclovir was stopped after treatment of a first episode of herpes there was a rapid recurrence of viral infection. However, when famciclovir was used instead (for five or 10 days) there was no recurrence when the drug was stopped. Clearly, if this were found to be true in humans, famciclovir would be the treatment of choice for first episodes of herpes simplex infection.[26] Unfortunately, the early suggestion that the use of famciclovir in first episodes might reduce recurrences has not, as yet, been confirmed. However, it remains unclear whether the magnitude of latency (that is, the number of latently infected neurons) can be limited by the prompt administration of nucleoside analogues or their prodrugs to such an extent that subsequent reactivations are less frequent.[27] A clinical trial is now underway to compare recurrence rates after treatment with valaciclovir and high dose famciclovir in a first episode of genital herpes.
Drugs against C trachomatis
Some drugs in the tetracycline class are generally considered to be the drugs of first choice for treating women with chlamydia infection who are not pregnant.[28] The preferred first line regimens are doxycycline 100 mg twice daily for seven days or Deteclo (a combination of three tetracycline drugs) 300 mg twice daily for seven days.[29] Concerns have been raised about the possible problems caused by lack of compliance with these regimens. In recent years, a number of studies have compared the microbial cure rates of doxycycline 100 mg twice daily and a single 1g dose of azithromycin. Most of these have shown higher cure rates in the doxycycline arm; others have shown no significant difference between the two arms. Two studies have shown microbial cure rates as low as 90%-92% for azithromycin.[30 31] Most of the studies have tested for a cure at a maximum of two weeks after the single dose of azithromycin. Some researchers have regarded this as too short an interval to be confident that a cure has been achieved. The high cure rates obtained with doxycycline imply that--at least in a trial setting-compliance is almost always sufficient As a consequence, the guidelines developed at the Royal College of Physicians relegated azithromycin to second line treatment (along with erythromycin, tetracycline, and ofloxacin).[29] It has been suggested that single dose treatment should be available for patients for whom compliance may be a problem.[32]
New techniques for managing chronic infection
HIV infection
Over the past year or two, viral load tests (to measure HIV RNA in serum) have become firmly established in clinical practice. The HIV viral load strongly predicts the rate of decrease in CD4 lymphocytes and progression to AIDS and death, and the combination of viral load plus CD4 count gives the most accurate prognosis.[33] These measurements, plus the clinical status of the patient, give the best guide to the controversial question of when to start antiretroviral treatment. The aim of treatment is, wherever possible, to suppress the viral load to below the level of detection of a sensitive assay (for example, [is less than] 50 copies/mi).
Dyskaryotic cervical smears
Human papillomavirus infection of the cervix is a common cause of dyskaryotic smears. However, in most cases human papillomavirus infection is transient. A recent study showed that the median duration of new human papillomavirus infections was eight months; hence conservative management, such as follow up without ablative treatment, may be indicated in young women with low grade squamous intraepithelial lesions associated with transient human papilloma virus infection.[34] Persistence of human papillomavirus for six months or more is related to older age, types of human papillomavirus associated with cervical cancer, and infection with several types of human papillomavirus.[34]
Another study examined women who had mild or moderate dyskaryosis on cervical smears and minor abnormalities seen at colposcopy.[35] Typing the human papillomavirus in these women predicts the risk of progressive disease. Those found to have human papillomavirus type 16 are at high risk of progression to cervical intraepithelial neoplasia grade 3 and those with other oncogenic types or non-oncogenic types are at low risk.[35]
Using a different protocol, a Dutch group was able to show that women with normal smears containing high risk human papillomavirus genotypes were 116 times (95% confidence interval 13 to 990 times) more at risk of developing cervical intraepithelial neoplasia grade 3 than women without high risk human papillomavirus.[36] The authors concluded that "these results support the view that the interval between successive smears in cervical cancer screening can be increased considerably for women with cytomorphologically normal and high risk human papillomavirus negative cervical smears as determined by polymerase chain reaction."
The view of the Department of Health is that "new screening programmes should not be introduced or expanded until reviewed, and proved effective" The current advice iS that "human papillomavirus testing should not be used at present in routine practice within the NHS Cervical Screening Programme or as an ad hoc screening test."[37]
I am grateful to Dr Chris Sonnex and Dr Stephen Kirker for their helpful comments.
Funding: None.
Conflict of interest: None.
[1] Simms I, Hughes G, Swan AV, Rogers PA, Catchpole M. New cases seen at genitourinary medicine clinics: England 1996. Commun Dis Rep CDR Supp 1998;8:S1-11.
[2] Adler MW.. Sexual health--a Health of the Nation failure. BMJ 1997;314:1743-7.
[3] Van de Laar MJW, van Duyhnoven YTHP, Dessens M, van Santen M, van Klingeren B. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the Netherlands, 1977-95. Genitourin Med 1997;73:512.
[4] Ison CA. Antimicrobial agents and gonorrhoea: therapeutic choice, resistance and susceptibility testing. Genitourin Med 1996;72:253-7.
[5] Fitzgerald M. Antibiotic treatment for gonorrhoea in the UK. Genitourin Med 1997;73:149.
[6] Van de Laar MJW, van Duynhoven YTHP, Dessens M, van Santen M, van Klingeren B. Surveillance of antibiotic resistance in Neisseria gonorrhoea in the Netherlands, 1977-95. Genitourin Med 1997;73:510-7.
[7] Lewis DA, Brook MG, Shaft MS. High level ciprofloxacin resistant gonorrhoea imported from Russia. Genitourin Med 1997;73:325-6.
[8] Tichonova L, Borisenko K, Ward H, Meheus A, Gromyko A, Renton A. Epidemics of syphilis in the Russian Federation; trends, origins, and priorities for control. Lancet 1997;350:210-3.
[9] Waugh MA. Epidemics of syphilis in the Russian Federation. Lancet 1997; 350:595.
[10] Battu VR, Horner PJ, Taylor PK, Jephcott AE, Egglestone SI. Locally acquired heterosexual outbreak of syphilis in Bristol. Lancet 1997;350:1100-1.
[11] Caul EO, Horner PJ, Leece J, Crowley T, Paul I, Davey-Smith G. Population-based screening programmes for Chlamydia trachomatis. Lancet 1997;349:1070-1.
[12] Stary A. Chlamydia screening: which sample for which technique? Genitourin Med 1997;73:99-102.
[13] Bulmer M, Van Doornum GJJ, Ching S, Peerbooms PGH, Plier PK, Ram D, et al. Detection of Chlamydia trachomatis and Neisseria gonorrhoea by ligase chain reaction-based assays with clinical specimens from various sites: implications for diagnostic testing and screening. J Clin Microbiol 1996;34:2395-400.
[14] Cowan FM, Johnson AM, Ashley R, Corey L, Mindel A. Relationship between antibodies to herpes simplex virus (HSV) and symptoms of HSV infection. J Infect Dis 1996;174:470-5.
[15] Ashley RL, Corey L. HSV type specific antibody tests: patients are ready, are clinicians? Genitourin Med 1997;73:235-6.
[16] Ross JDC, Smith IW, Elton PA. The epidemiology of herpes simplex types 1 and 2 infection of the genital tract in Edinburgh 1978-91. Genitourin Med 1993;69:381-3.
[17] Tayal SC, Pattman RS. High prevalence of herpes simplex virus type 1 in female anogenital herpes simplex in Newcastle-upon-Tyne 1983-92. Int J STD AIDS 1994;5:359-61.
[18] Edwards S, White C. Genital herpes simplex virus type 1 in women. Genitourin Med 1994;70:426.
[19] Fairley I, Monteiro EF. Patient attitudes to type specific serological tests in the diagnosis of genital herpes. Genitourin Med 1997;73:259-62.
[20] Coker DM. HSV type specific antibody tests. Genitourin Med 1997;73:580.
[21] Volberding PA. Protease inhibitors vindicated. Lancet 1997;350 (suppl 111):10.
[22] Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimetre or less. N Engl J Med 1997; 337:725-33.
[23] Bodsworth NJ, Crooks RJ, Borelli S, Veglsgaard G, Paavonen J, Worm A-M, et al. Valaciclovir versus aciclovir in patient initiated treatment of recurrent genital herpes: a randomised, double blind clinical trial. Genitourin Med 1997;73:110-6.
[24] Patel R, Bodsworth NH, Woolley P, Peters B, Veglsgaard G, Saari S, et al. Valaciclovir for the suppression of recurrent genital HSV infection: a placebo controlled study of once daily therapy. Genitourin Med 1997;73; 105-9.
[25] Field HJ, Tewari D, Sutton D, Thackray AM. Comparison of efficacies of famciclovir and valaciclovir against herpes Simplex virus type 1 in a murine immunosuppression model. Antimicrob Agents Chemother 1995;39:1114-9
[26] Ahmed A, Woolley PD. Comparison of famciclovir and valaciclovir in first episode genital herpes: possible clinical effect of latency. Proceedings of the Eurogin conference on herpes viruses and genital pathology, Paris, 1996.
[27] Simmons A, Field HJ. Can HSV latency be conquered by current antiviral therapies? Sex Transm Inf 1998;74:1-2.
[28] Taylor-Robinson D. Chlamydial infection in pregnancy. Continuing Medical Education Bulletin. Sexually Transmitted Infections and HIV. 1997;1:37-40.
[29] FitzGerald MR, Welch J, Robinson AJ, Ahmed-Jushuf IH. Clinical guidelines and standards for the management of uncomplicated genital chlamydial infection. Int J STD AIDS 1998;9:253-62.
[30] Lauharanta J, Saarinen K, Mustonen MT, Happonen HP. Single-dose oral azithromycin versus seven-day doxycycline in the treatment of non-gonococcal urethritis in males. Antimicrob Chemother 1993;31(suppl E):177-83.
[31] Steingrimsson O, Olafsson JH, Thorarinsson H, Ryan RW, Johnson RB, Tilton RC. Single dose azithromycin treatment of gonorrhoea and infections caused by C trachomatis and U urealyticum in men. Sex Transm Dis 1994;21:43-6.
[32] Hillis SD, Coles FB, Litchfield B, Black CM, Mojica B, Schmitt K, et al. Doxycycline and azithromycin for prevention of chlamydial persistence or recurrence one month after treatment in women. Sex Transm Dis 1998;25:5-11.
[33] Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and [CD4.sup.+] lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997;126;946-54.
[34] Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338:423-8.
[35] Londesborough P, Ho L, Terry G, Cuzick J, Wheeler C, Singer A. Human papillomavirus genotype as a predictor of persistence and development of high-grade lesions in women with minor cervical abnormalities. Int J Cancer 1996;69:364-8.
[36] Rozendaal L, Walboomers JMM, Van Der Linden JC, Voorhorst FJ, Kenemans P, Helmerhorst ThJM, et al. PCR-based high-risk HPV test in cervical cancer screening gives objective risk assessment of women with cytomorphologically normal cervical smears. Int J Cancer 1996;68:766-9.
[37] Department of Health. Message from the Chief Medical Officer to Medical Directors. London: Doll, 1998. (CEM/CMO/98/3.)
(Accepted 16 April 1998)
RELATED ARTICLE: Recent advances
Amplification assays, such as the ligase chain reaction, have excellent sensitivity and specificity
A third class of anti-retroviral drug is now available--the non-nucleoside reverse transcriptase inhibitors, such as nevirapine
Valaciclovir and famciclovir offer more convenient dosage regimens than aciclovir
A single dose regimen of azithromycin has a place in treating Chlamydia trachomatis, especially where compliance may be a problem
Viral load assays for HIV are the best way of predicting disease progression and monitoring anti-retroviral treatment
Typing of the human papillomavirus can predict the risk of progressive disease in women with normal, mild, and moderately dyskaryotic vaginal smears
RELATED ARTICLE: Fifty years ago The new NHS: National Health Service Act
In 1942 the Medical Planning Commission of the British Medical Association produced a valuable draft interim report, and Sir William Beveridge's Report on Social Insurance and Allied Services appeared. Following on this came the White Paper entitled "A National Health Service" (Cmd. 6502), published in 1944, in which the Coalition Government made proposals for the establishment of such a service. In 1946 Parliament passed the National Health Service Acts, by which the Minister of Health and the Secretary of State for Scotland are to promote a comprehensive health service for the improvement of the physical and mental health of the people of England and Wales and Scotland, and for the prevention, diagnosis, and treatment of illness. Thus, in the course of a hundred years the work of preventive and social medicine, work for the most part, as we have seen, inspired and directed by the medical profession, comes to full fruition.
In new ways and with new methods the medical profession will carry on the great traditions of their predecessors in the promotion of national health. As Disraeli said in 1877, "The health of the people is really the foundation upon which all their happiness and all their powers as a State depend." (3 July 1948, p 9. See also editorial by Gordon Macpherson, 3 January 1998, p 6.)
Department of Genitourinary Medicine, Addenbrooke's Hospital, Cambridge CB2 2QQ Chris Came, consultant
christopher, carne@ msexc.addenbrookes. anglox.nhs.uk
BMJ 1998;317:129-32
COPYRIGHT 1998 British Medical Association
COPYRIGHT 2000 Gale Group