HUMAN EXPOSURE TO ETHYLENE OXIDE (EtO) occurs mainly through inhalation of polluted air (1,2) in occupational workplaces and/or via tobacco smoke. (3) EtO is also produced within the human body as a metabolic activation product of ethylene, (4) which is a common urban air pollutant (5,6) and a component of the gaseous phase of tobacco smoke. (3) Epidemiological studies in occupationally EtO-exposed humans have shown that this epoxide represents a definite risk factor for leukemia and a possible risk factor for solid tumors. These risks represent 1 component of the overall carcinogenic risk to humans of tobacco smoke (both active and passive). (6-9)
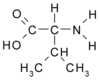
EtO is an electrophilic compound that reacts easily with deoxyribonucleic acid (DNA) and proteins, without metabolic activation. (10,11) The major reaction product in DNA is N-7(2-hydroxyethyl)guanine, and major reactive sites in hemoglobin are cysteine, histidine, and in particular N-terminal valine. (12) The molecular adduct of EtO with valine, namely N-(2-hydroxyethyl)valine (HOEtVal), represents a biological effective dose marker for EtO arising from both direct intake and the metabolization of ethylene. (13-16)
In this study, we sought to measure the extent of HOEtVal formation in the hemoglobin of 360 healthy adults who were not occupationally exposed to EtO, and to relate this biochemical parameter to the smoking habits of each subject. The subjects' smoking habits were determined from a questionnaire, and urinary cotinine was used as an internal dose marker for exposure to tobacco smoke. (17)
Materials and Method
Choice of subjects. Study subjects (N = 360; all > 18 yr of age) were recruited randomly from the Italian Association of Blood Donors in the city of Torino (Turin) in northwestern Italy, from December 1996 to February 1997. The only selection process criterion adopted was the enrollment of a similar number of males and females. For each subject, the study procedure entailed administration of a questionnaire and collection of a venous blood sample and a urine sample.
Data collection, analysis, and quality control
Questionnaire. The survey questionnaire, administered by the same interviewer for all subjects, contained several questions in each of the following areas: personal (sex, age, residence, smoking habits); familial (number of cohabitants and smoking habits of each); and profession. The questionnaire was readministered 1 mo later to 50 subjects, by the same interviewer, and good agreement was seen between replicated replies.
Blood samples. From each subject, 5 ml of venous blood was collected in heparinized tubes and processed within 4 hr, as described previously. (16) The globin fraction was isolated and derivatized with pentafluorophenyl isothiocyanate (PFPITC). (13,14) In particular, the modified globin containing a terminal hydroxyethylvaline residue underwent derivatization, and N-(2-hydroxyethyl)valine-pentafluorophenyl-thiohydantoin (HOEtVal-PFPTH) was the final product of interest. A calibration curve was prepared, using a calibration standard, and an internal standard was obtained by alkylating in vitro (with EtO and deuterated EtO [Et[O.sub.2][d.sub.4]], respectively) a blood sample obtained from a nonsmoker subject (background standard globin). The degree of alkylation of these background standard globins was calculated from their chromatographic signals: calibration standard = 6.3 [micro]mol/gm of globin; internal standard = 5.35 [micro]mol/gm of globin. (16) Gas chromatography/mass spectrometry (GC/MS) analysis was performed with a Finnigan-MAT 95 Q (Bremen, Germany) mass spectrometer in the electron-capture negative chemical ionization (ECNI) mode. Results for the quality control procedures are described in detail elsewhere. (16) In brief: the detection limit was 10 pmol of HOEtVal/gm of hemoglobin. The coefficient of variation (CV%) for reproducibility of the internal standard was 6.2%, and for samples was 3.5%. The correlation coefficient ([r.sup.2]) of the calibration curve was .999300 (y = .000946).
Urine samples. A 25-ml sample of "morning" urine was obtained from each subject. The urine samples were stored for 1 mo (or less) at-70[degrees]C until analysis. Quantitative analysis of urinary cotinine was performed by GC with nitrogen, phosphor detection. Preparation of the samples and the calibration curve, GC analysis, and quality control were described previously. (17)
Statistical analysis. Statistical analysis was performed using SPSS Packages, version 8.0. (18)
Results and Discussion
Table 1 describes the subjects involved in the study by gender, age, and smoking habits. The study population was a priori divided into 3 subcategories of individuals, according to their smoking habits: (1) nonsmokers; (2) passive smokers (i.e., nonsmoking individuals living or working with active smokers); and (3) active smokers, as determined from the questionnaire.
Quantitative results for HOEtVal and urinary cotinine are reported in Table 2. It is evident that active exposure to tobacco smoke corresponded to higher concentrations of the 2 biological markers.
Figures 1 and 2 show the linear regressions between the number of cigarettes smoked per day and, respectively, HOEtVal and urinary cotinine for all 360 subjects. The number of cigarettes actively and passively smoked per day was derived from the questionnaire. The 2 statistical analyses showed significant correlations ([r.sup.2] = .4416 and .5893, respectively), thus demonstrating a close relationship between exposure to cigarette smoke and each of the 2 biological markers. The linear regression calculated directly between the 2 markers is shown in Figure 3. A positive correlation coefficient ([r.sup.2] = .3893) is obtained, supporting the positive correlation between cotinine concentration in urine and HOEtVal in the blood hemoglobin formed by repeated exposure to ethylene and EtO arising from tobacco smoke. As a consequence of this correlation, the determination of cotinine can be used to approximate the early biological effects of tobacco smoke exposure. It is worth noting that both the latter correlation and the personal evaluation of the number of cigarettes smoked per day are free from the possible bias inherent in a subjective index.
[FIGURES 1-3 OMITTED]
The linear regression between the 2 biomarkers, calculated for the 360 study subjects (Fig. 3), allows one to estimate that 5 ng/ml of cotinine corresponds to 1 pmol/gm of HDEtVal. When only the active smokers are considered, this correspondence increases to 10 ng/ml for 1 pmol/gm, whereas for nonsmokers it is 2 ng/ml for 1 pmol/gm of HOEtVal. Table 3 shows the HOEtVal values (y') estimated by a regression analysis for both smokers ([y'.sub.s]) and nonsmokers ([y'.sub.ns]).
Ratios between the HOEtVal values estimated in smokers on the basis of the urinary cotinine values were 1.04 or 1.03. This increase in the estimated value was approximately 3-4%, for a constant increase of 20 ng/ml of urinary cotinine. In contrast, the ratios between the HOEtVal values estimated in nonsmokers from the next urinary cotinine values were 1.41, 1.29, 1.23, and 1.1 8. This increase in the estimated value was 41%, 29%, 23%, and 18%, respectively, for a constant increase of 20 ng/ml of urinary cotinine. The estimated HOEtVal values in nonsmokers were higher than those in smokers--for the same increase in urinary cotinine. Furthermore, the estimated urinary cotinine value in nonsmokers was higher than that estimated for smokers at a urinary cotinine level of 100 ng/ml. Apparently, HOEtVal reaches a saturation value for high exposure to tobacco smoke. This effect cannot be ascribed to the determination method, because the calibration curve extends far beyond the saturation value.
Table 4 shows the single Pearson's correlations measured between the number of cigarettes per day vs. cotinine vs. HOEtVal. These relationships were significant for active smokers, but not for passive smokers.
Figure 4 reports the means and 95% confidence intervals of HOEtVal in the 3 study groups, together with results of analysis of variance (ANOVA). The general model exhibited a high significance (p = .0001), confirming the nonhomogeneous distribution of the 3 groups of concentrations. Taking nonsmokers as the reference category, the entire distribution difference evidenced in the model can be ascribed to the group of active smokers. An analogous ANOVA test was performed for urinary cotinine. In this case as well, the general model proved highly significant ([F.sub.2, 357] = 98.32; p = .0001; [r.sup.2] = .355), and the difference was entirely ascribed to the group of active smokers.
[FIGURE 4 OMITTED]
The results relative to both HOEtVal and urinary cotinine in adults did not distinguish between passive smokers and nonsmokers, nor could we determine differences within the group of passive smokers (Fig. 4). Nevertheless, several researchers have demonstrated that cotinine can discriminate among passive smokers with high sensitivity. In a previous study, we demonstrated that the concentration of urinary cotinine determined for a large group of adolescents correlated linearly with the passive exposure to tobacco smoke declared in a questionnaire. (17) Perhaps the correlation observed between urinary cotinine and passive smoking in adolescents, but not in adults, reflected the simpler lifestyle of young nonsmokers (14 yr of age), who are exposed almost exclusively to the tobacco smoke of their cohabitants. In contrast, the complex and variable lifestyle of the adult population increases the chance of unaware passive exposure to tobacco smoke. Consequently, several subjects included in the nonsmokers group of this study were likely to be unaware passive smokers. Thus, a bias may have arisen from subjective declarations, whose main effects consist in enlarging the distribution of cotinine values for the group of nonsmokers, resulting in the absence of correlation observed in Table 4. The same bias justifies the lack of difference in HOEtVal levels between passive smokers and nonsmokers.
A multivariate analysis was conducted with HOEtVal as the dependent variable, and several grouping parameters (from the questionnaires) were independent variables. The results, shown in Table 5, confirm the conclusions obtained by univariate analysis: only gender and active exposure to tobacco smoke have a direct influence on the formation of HOEtVal.
Conclusions
N-(2-hydroxyethyl)valine and cotinine represent 2 important biomarkers whose correlation may correlate exposure to tobacco smoke with the risk of tumor onset in the following ways: (a) recent tobacco smoke exposure is detected with high sensitivity and specificity by urinary cotinine (half-life = 20 hr), making it an internal dose marker; and (b) early biological effects are detected by the level of HOEtVal, a molecular adduct of EtO to hemoglobin, representing a longer "expositive history" (half-life = 60 days).
The positive correlation between urinary cotinine and HOEtVal ([r.sup.2] = .3893) can be reasonably extrapolated to the corresponding DNA adduct. Thus, each of these parameters may signal the occurrence of some early stages of the carcinogenic mechanisms related to active exposure to tobacco smoke. In fact, the level of HOEtVal correlates linearly with its alkylating activity in DNA. In particular, Farmer calculated that 10 pmol of HOEtVal/gm of globin corresponds to 0.33 pmol of adduct/gm of DNA. (19) Thus, it follows that the determinations of cotinine and HOEtVal could find practical application in estimating the risk of cancer related to tobacco smoking.
In future studies we will look more closely at the group of passive tobacco smokers and use the 2 biomarkers. To avoid the possible bias associated with subjective declarations, we plan to conduct a new epidemiological study that considers a large group of adolescents, whose lifestyle we assume would be more homogeneous than that of adults. This new study will require an increased sensitivity in HOEtVal analysis, achieved by using a tandem mass spectrometric detection technique to attain a detection limit of 1 pmol/gm.
This study was made possible by a grant of from Ministero dell' Universita, della Ricerca Scientifica e Tecnologica, 60% 1996 and 1997; from ISPESL 1999; and from Fondazione CRT of Torino 1998.
This study was conducted in accordance with national and institutional guidelines for the protection of human subjects, and informed consent was obtained for each subject.
Submitted for publication July 14, 2000; revised; accepted for publication April 16, 2001.
Requests for reprints should be sent to Professor Roberto Bono, Department of Public Health and Microbiology, University of Torino, via Santena 5 bis, 10126 Torino, Italy.
E-mail: roberto.bono@unito.it
References
(1.) Osterman-Golkar S, Bergmark E. Occupational exposure to ethylene oxide: relation between in vivo dose and exposure dose. Scand J Work Environ Health 1988; 14:372-77.
(2.) Ribeiro LR, Salvadori DMF, Rios ACC, et al. Biological monitoring of workers occupationally exposed to ethylene oxide. Mutat Res 1994; 313:81-87.
(3.) Guerin MR. Formation and physicochemical nature of sidestream smoke. In: O'Neill IK, Brunnemann KD, Dodet B, Hoffmann D (Eds). Environmental Carcinogens. Methods of Analysis and Exposure Measurement, vol 9, Passive Smoking. International Agency for IARC Sci Publ, No. 81. Lyon, France: IARCPress, 1987; pp 11-23.
(4.) Segerback D. Alkylation of DNA and haemoglobin in the mouse following exposure to ethene and ethene oxide. Chem Biol Interact 1983; 45:139-51.
(5.) Sawada S, Totsuka T. Natural and anthropogenic sources and fate of atmospheric ethylene. Atmos Environ 1986; 20:821-32.
(6.) International Agency for Research on Cancer (IARC). Ethylene oxide. In: Some Industrial Chemicals. IARC Monogr Eval Carcinog Risks Hum, vol 60. Lyon, France: IARCPress, 1994; p 73.
(7.) International Agency for Research on Cancer (IARC). Tobacco Habits Other than Smoking; Betel-Quid and Areca-Nut Chewing; and Some Related Nitrosamines. IARC Monogr Eval Carcinog Risks Hum, vol 37. Lyon, France: IARCPress, 1985; 291 pp.
(8.) International Agency for Research on Cancer (IARC). Tobacco Smoking. IARC Monogr Eval Carcinog Risks Hum, vol 38. Lyon, France: IARCPress, 1986; 421 pp.
(9.) U.S. Environmental Protection Agency (EPA). Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders (EPA/600/6-90/006F), 1993. Available from EPA National Center for Environmental Publications, Cincinnati, OH; EPA-43-F-93-004.
(10.) Osterman-Golkar S, Farmer PB, Segerback D, et al. Dosimetry of ethylene oxide in the rat by quantitation of alkylated histidine in hemoglobin. Teratog Carcinog Mutagen 1983; 3:395-405.
(11.) Segerback D. Reaction products in hemoglobin and DNA after in vitro treatment with ethylene oxide and N-(2-hydroxyethyl)-N-nitrosourea. Carcinogenesis 1990; 11:307-12.
(12.) Bryant MS, Osterman-Golkar S. Hemoglobin adducts as dosimeters of exposure to DNA-reactive chemicals. Chemical Industry Institute of Toxicology, Research Triangle Park, NC. CIIT Activities 1991; 11(10):1-12.
(13.) Tornqvist M, Mowrer J, Jensen S, et al. Monitoring of environmental cancers initiators through hemoglobin adducts by a modified Edman degradation method. Anal Biochem 1986; 154: 255-66.
(14.) Tornqvist M. The N-alkyl Edman method for hemoglobin adduct measurement: updating and applications to humans. In: Human Carcinogen Exposure: Biomonitoring and Risk Assessment. Oxford, UK: Oxford University Press, 1991; pp 411-19.
(15.) Tornqvist M. Epoxide adducts to N-terminal valine of hemoglobin. In: Everse J, Winslow RW, Vandegriff KD (Eds). Methods in Enzymology, vol 231. San Diego, CA: Academic Press, 1994; pp 650-57.
(16.) Bono R, Vincenti M, Meineri V, et al. The formation of N(2-hydroxyethyl)valine due to exposure to ethylene oxide via tobacco smoke: a risk factor for onset of cancer. Environ Res 1999; A81: 62-71.
(17.) Bono R, Russo R, Arossa W, et al. Involuntary exposure to tobacco smoke in adolescents: urinary cotinine and environmental factors. Arch Environ Health 1996; 51(2):127-31.
(18.) SPSS. Packages, vers 8.0. Cary, NC: SPSS, Inc., 1998.
(19.) Farmer PB, Neumann HG, Henschler D. Estimation of exposure of man to substances reacting covalently with macromolecules. Arch Toxicol 1987; 60:251-60.
COPYRIGHT 2002 Heldref Publications
COPYRIGHT 2003 Gale Group