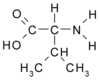
ABSTRACT. Background: Alanine and glutamine are released from muscle in response to critical illness. Subsequent depletion of glutamine from muscle is proposed as a principal factor in the limitation of muscle protein synthesis in severely ill patients. The objective of this study was to assess the peripheral metabolic response to enteral supplementation of alanine, glutamine, and valine in critically ill patients. Methods: Isotopic tracers of alanine, glutamine, and phenylalanine were given IV to 6 critically ill patients and 6 healthy volunteers. Blood sampling from the femoral artery and vein along with muscle biopsies provided assessment of leg (ie, muscle) kinetics. Measurements were obtained during enteral nutrition alone and then with combined alanine (11.25 g), glutamine (7.5 g) and valine (11.25 g) supplementation for 3 hours. Results: Compared with healthy volunteers, critically ill patients had significantly reduced concentrations of alanine and glutamine in arterial plasma (p
Critical illness is associated with the substantial release of glutamine from muscle and a rapid depletion of the intramuscular free glutamine pool.1 Rennie et al2 and Hammarqvist et al have found a strong relationship between reductions in the free glutamine concentration within muscle and limitations in the rate of muscle protein synthesis. Because the rate of muscle catabolism typically exceeds synthesis during severe illness, these researchers theorized that maintenance of glutamine concentration within muscle may augment and preserve net protein synthesis during catabolic conditions and thus minimize or possibly negate muscle wasting. Unfortunately, recent work demonstrates that massive supplementation of glutamine to critically ill patients does not replenish glutamine availability within muscle, apparently from a failure of glutamine transport into muscle.4
During critical illness, alanine release from muscle is accelerated to a greater extent than any other amino acid.5 Because pyruvate availability is rate limiting in the synthesis of alanine under a variety of conditions, accelerated glycolysis and the subsequent production of pyruvate appears a principal factor in driving the increased production and release of alanine during catabolic stress (Fig. 1).6,7 Once formed, pyruvate is transaminated with glutamate to produce alanine and [alpha]-ketoglutarate. Glutamate is also an essential substrate in the production of glutamine. Thus, increases in alanine production may reduce glutamate availability and thereby limit the capacity of muscle to replenish glutamine.
The purpose of this study was to assess if glutamine production within muscle could be stimulated by a combination of alanine (to inhibit glutamate transamination with pyruvate), glutamine (in the hope of reducing glutamine transport out of muscle), and valine (to increase nitrogen availability and thus support glutamine production from [alpha]-ketoglutarate). Furthermore, in anticipation that this amino acid mixture could augment glutamine production and replenish the intramuscular concentration of glutamine, an additional study objective was to determine the effect of repletion of muscle glutamine on muscle protein kinetics. Such information maybe of clinical significance in assessing the benefit, if any, of amino acid supplementation on the preservation of lean body mass during severe illness.
MATERIALS AND METHODS
Study Subjects
Characteristics of the study subjects are presented in Table I. The 6 normal volunteers (all men) were deemed healthy by medical history, physical examination, and screening laboratory profile. Their average age was 31 years + or - 3 (mean + or - SEM), and they were normal for height and weight (86 kg + or - 5) and were not taking any medications. At the time of study, the 6 critically ill patients required prolonged mechanical ventilation for nosocomial pneumonia and were deemed septic by consensus conference definition.8 All had surgical procedures >10 days before study but within the past 4 weeks. These patients were receiving enteral nutrition via an indwelling nasal jejunal tube for a minimum of 10 days before study. Five of the 6 patients were men with an average age of 57 years + or - 10 and with an average weight of 87 kg + or - 4. Acute Physiology and Chronic Health Evaluation II scores averaged 22 + or - 5.
Study Protocol
This study was approved by the institutional review board of The University of Texas Medical Branch in Galveston, Texas. Informed written consent was obtained from the volunteers and from a family member of each patient before study. The study protocol is diagrammed in Figure 2. Healthy volunteers were admitted to the Clinical Research Center. Patients were studied while remaining in their intensive care unit rooms. Local anesthesia (1% lidocaine) facilitated placement of catheters into an adjacent femoral artery and vein and in two upper extremity veins for the normal volunteers. For the patients, an indwelling central venous access was used in preference to an extremity IV. Normal volunteers drank 250 mL of Peptamen with ice 1 hour before study and then 80 mL of Peptamen hourly for the remainder of the experiment. For all patients, enteral nutrition was standardized to Peptamen at 80 mL per hour beginning at midnight before study and continued through the study. Peptamen was used in this study, being chosen previously by clinicians as the preferred enteral supplement for intensive care patients. The nutritional characteristics of Peptamen are presented in Table II. Between the third and fourth hour of study, leg blood flow was determined by infusion of indocyanine green dye (1 mg/min x 20 min) into the femoral artery, with subsequent spectrophotometric analysis of blood drawn simultaneously from the femoral vein and from the peripheral or central line access.9 Leg blood flow measurements were standardized for leg volume as assessed by integrating several circumference measurements with the length of the calf, thigh, and foot. After baseline blood sampling, d^sub 3^ alanine (35 umol/kg prime; infusion at 0.35 umol/kg/min), 5^sup 15^N glutamine (63 umol/kg prime; infusion at 0.35 umol/kg/min), and d^sub 5^ phenylalanine (2 umol/kg prime; infusion at 0.07 umol/kg/min) were given via the upper extremity or central venous access for the 8 hours of study. Under local anesthesia, biopsies were obtained from the vastus lateralis muscle using a Bergstrom needle at the second and fifth hours of study. At 4:30, 4:45, and fifth hour, blood was drawn simultaneously from the femoral artery and vein thus completing the 5-hour basal period.
Immediately after basal-period measurements, a drink of 5.625 g alanine, 3.75 g glutamine, 5.625 g valine, and ice water was given orally to the volunteers. Those volunteers subsequently drank this same amount of amino acid/ice water mixture; one half at the sixth hour of study and the remainder at the seventh hour. Thus, a total of 11.25 g of alanine, 7.5 g glutamine, and 11.25 g of valine were ingested by volunteer subjects for the final 3 hours of study (ie, amino acid period). Furthermore, the volunteers continued to drink 80 mL of Peptamen hourly. The critically ill patients received the 11.25 g alanine, 7.5 g glutamine, and 11.25 g valine (10% solution with water) as a continuous enteral infusion during the final 3 hours of study (amino acid period). This unlabeled amino acid mixture was delivered via the nasal jejunal tube along with the continued administration of Peptamen. Leg blood flow and serial blood sampling were again performed over the final 30 minutes of study, and a repeat muscle biopsy at the eighth hour completed the amino acid period and the experiment.
Sample Analysis
Blood concentrations of unlabeled alanine, glutamine, and phenylalanine and isotopic enrichment of each amino acid were simultaneously determined by gas chromatography, mass spectrometry (GCMS) using an internal standard method.10 Blood was collected in ice-cold tubes containing 2 mL of 15% sulfosalicylic acid and 200 [mu]L of internal standard (^sup 13^C phenylalanine, N^sup 15^ alanine, d^sub 5^ glutamine). Samples were vortexed and centrifuged. The supernatant was then frozen at -80[degrees] until processing. After tert-butyl dimethyl sialyl derivation, plasma samples were analyzed by means of GCMS (GCMS 5989; Hewlett Packard, Palo Alto, CA) using electron impact ionization.11
Muscle biopsies were analyzed for both intracellular and protein-bound amino acid concentrations and isotopic enrichment also using an internal standard method and analysis by GCMS.12 To approximately 20 mg of muscle were added 800 mL of 14% perchloric acid and 2 [mu]L of internal standard. Samples were homogenized and centrifuged, and the supernatant was collected. This procedure was repeated twice more and the pooled supernatant processed identically to the blood samples using tert-butyl dimethyl sialyl derivation with analysis by GCMS. This method determined the free intracellular concentration and isotopic enrichment of the 3 amino acids (alanine, glutamine, phenylalanine). For determination of protein-bound enrichment and concentration, the remaining muscle pellet was washed repeatedly with saline and absolute ethanol, dried, and then hydrolyzed with 6-N HC1.13 The protein hydrolysate was then passed over a cation exchange column (Dowex AG; BioRad Laboratories, Richmond, CA), dried, esterified, heated, and subsequently analyzed by GCMS using chemical impact ionization as previously described.13
Calculations
The net balance of alanine, glutamine, and phenylalanine across the leg was calculated as follows and expressed per 100 mL leg volume:14
AV NB = ([AA]art - [AA]v)LBF
where AV NB = amino acid net balance across the leg (nmol/min/100 mL leg volume), [AA]art, v = concentration of each amino acid from artery, vein (nmol/mL), and LBF = leg blood flow (ml/min/100 mL leg volume). Thus, a negative net balance reflects the net release of that amino acid from the limb.
The kinetics of alanine, glutamine, and phenylalanine within skeletal muscle were calculated using a 3-pool model as previously described in detail.14 This model allows quantification of amino acid transport into and out of muscle and muscle protein synthesis and breakdown. Values are calculated as follows:
Fin = [AA]art x LBF
Fout = [AA]v x LBF
Fma = [(IEm - IEv)/(IEart - IEm)] [AA]v + [AA]art) LBF
Fvm = [(IEm - IEv)/(IEart - IEm)] [AA]v + [AA]v) LBF
Fmo = Fma (IEart/IEm - 1)
Fom = Fmo + net balance
where: Fin = amino acid flow into leg (nmol/min/100 mL leg vol)
Fout = amino acid flow out of leg (nmol/min/100 mL leg vol)
Fma = flow of amino acid from artery into muscle (nmol/min/100 mL leg vol)
Fvm = flow of amino acid from muscle to vein (nmol/min/100 mL leg vol)
Fmo = release of amino acid from protein-bound to free concentration in muscle (nmol/min/100 mL leg vol)
Fom = disappearance of amino acid from the intracellular pool (nmol/min/100 mL leg vol)
Phenylalanine is an essential amino acid, and release from muscle can only come from breakdown of muscle protein. Thus, Fmo for phenylalanine reflects the rate of muscle protein breakdown.14 Likewise, Fom for phenylalanine represents the rate of muscle protein synthesis because there is no other fate for the irreversible disappearance of phenylalanine in muscle. However, alanine and glutamine are nonessential amino acids, and thus both muscle protein breakdown and de novo synthesis can contribute to the production of each amino acid. Therefore, the calculations for the rate of de novo synthesis of alanine and glutamine from muscle are indexed by the rate of appearance of phenylalanine.10 This approach assumes that the release of an amino acid from muscle proteolysis is proportional to its abundance in muscle protein. For alanine, the Fmo for phenylalanine was multiplied by 2.8 because each 100 g of muscle protein contains approximately 23 mmol of phenylalanine and 65 mmol of alanine. For glutamine, the Fmo for phenylalanine was multiplied by 2 because each 100 g of muscle protein contains approximately 46 mmol of glutamine. This estimated value for the release of each amino acid from muscle protein breakdown is subtracted from the rate of intracellular appearance (Fmo) for determination of the rate of amino acid synthesis from muscle.
Muscle de novo synthesis rate (nmol/min/100 mL leg vol)
for alanine = Fmo Ala - (2.8) (Fmo Phe)
for glutamine = Fmo Gln - (2) (Fmo Phe)
The 3-pool model as discussed above provides quantitation of muscle protein synthesis. However, as an independent measure of muscle protein synthesis, the fractional synthesis rate was determined by quantifying the rate of incorporation of d^sub 5^ phenylalanine into muscle over time and is calculated as follows:15
FSR = [(IE p^sub 2^ - IE p^sub 1^)/(IEm x t)] x 60 x 100
where FSR = fractional synthesis rate (%/h)
IE p^sub 2^, IE p^sub 1^ = isotopic enrichment of protein-bound d^sub 5^ phenylalanine in muscle at times 1, 2
IEm = average isotopic enrichment in muscle total parenteral nutrition = time (min)
factors 60,100 are required to express FSR in percentage/hour.
Statistics
Student's independent t test was used for statistical comparison of results between critically ill patients and volunteers. Student's paired t test was used to assess the relevance of any variations between basal and amino acid period measurements for both critically ill patients and volunteers. Results are presented as mean + or - SEM and p
RESULTS
Although the volunteers were similar in age (range, 23-34 years), the patients ranged from 22 to 72 years (Table I). Furthermore, the patients were slightly older on average than the volunteers. The healthy volunteers and critically ill patients were not statistically different in weight. Statistical regression analysis failed to identify any substantial variation in metabolic measurements with the age or weight of the patients. The patients were hypermetabolic, as evidenced by their increased energy expenditure and mild hyperglycemia (Table I). Over 2 weeks after study, 2 of the 6 patients died of multisystem organ failure; all of the healthy volunteers survived study participation.
Arterial plasma concentrations of alanine and glutamine were significantly less in the critically ill patients compared with the normal volunteers (Table III). Ingestion of the amino acid mixture significantly increased arterial plasma amino acid concentrations for both alanine and glutamine. Arterial plasma concentration of phenylalanine was not substantially affected by the alanine, glutamine, and valine intake. Concentrations of alanine in muscle were similar between patients and volunteers and not appreciably altered by the ingestion of the amino acid mixture (Table III). Muscle concentrations of free glutamine were significantly less in the severely ill patients compared with the normal healthy volunteers, with no overt change in the muscle concentration of glutamine in the patients or volunteers after the ingestion of the amino acid mixture. Phenylalanine concentration in muscle was significantly higher in the patients compared with the healthy volunteers. There was no substantial change in the muscle concentration of phenylalanine with the ingestion of the 3 amino acids.
Leg net balance of alanine was similar between critically ill patients and healthy volunteers (Table IV). Patients differed from volunteers by having significantly faster rates of transport of alanine into and out of muscle and increased rates of incorporation and production of alanine into and from muscle protein. Furthermore, patients had a significantly higher rate of de novo synthesis of alanine from muscle. For the patients, the supplementation of alanine, glutamine, and valine decreased the negative net balance for alanine across the leg. Amino acid administration was also associated with increased flux of alanine into and out of the leg for both patients and volunteers. Rates of production and consumption of alanine within muscle were not substantially affected by amino acid supplementation for either the patients or the volunteers.
Despite the discrepancy in the intracellular concentration of glutamine, the net balance of glutamine was similar for critically ill patients and volunteers (Table V). However, transport of glutamine into and out of muscle was significantly less for the patients, as were the rates of glutamine incorporation into protein and the production of glutamine from muscle. Furthermore, de novo synthesis of glutamine within muscle was also significantly less for patients compared with volunteers. For the patients and for the volunteers, amino acid administration failed to alter arterial-venous net balance and transport of glutamine into and out of muscle. Furthermore, the rates of glutamine incorporation into and production from muscle protein were not significantly affected for either the patients or the healthy volunteers.
There was a significantly greater net efflux of phenylalanine from the leg in the critically ill patients compared with the healthy volunteers (Table VI). Furthermore, transport of phenylalanine into and out of muscle was significantly greater in the patients compared with volunteers. As an index of muscle protein metabolism, the rate of phenylalanine incorporation into muscle (ie, muscle protein synthesis) and the rate of phenylalanine release from muscle protein breakdown were significantly increased in patients compared with volunteers. Supplementation of alanine, glutamine, and valine failed to alter muscle protein metabolism in either the patients or the volunteers. In addition to the 3-pool model measurements, quantifying the isotopic enrichment of phenylalanine in sequential muscle biopsies provided quantitation of the fractional synthetic rate of muscle protein. This measurement demonstrated a significantly increased rate of muscle protein synthesis in the patients compared with the volunteers (Fig. 3). There was no significant change the fractional synthetic rate of muscle protein with the ingestion of the amino acid mixture in either the patients or the volunteers.
DISCUSSION
Many of the metabolic consequences of prolonged critical illness on muscle protein were evident in the study patients. For example, de novo glutamine production was reduced, as were the rates of glutamine incorporation into and appearance from muscle in severely ill patients. These factors reaffirm the concept that limitations in glutamine production underlie the depletion of glutamine with prolonged critical illness.16 Such findings have fostered the concept of glutamine as a conditionally essential amino acid in prolonged stress conditions with detrimental clinical consequences on immune function and wound healing.17 Several studies demonstrate that alimentation of nutrition support with glutamine is beneficial by reducing septic morbidity in .catabolic patients.18,19 Furthermore, Rennie et al2 postulated that the concentration of free glutamine in muscle appears to regulate the rate of muscle protein synthesis. Thus, the significant reduction in glutamine concentration within muscle during prolonged illness may limit the synthetic capacity of muscle protein and thereby result in a greater net catabolism of lean body mass. Therefore, maneuvers that would replenish glutamine availability in muscle could theoretically benefit in augmenting net muscle protein synthesis and thus possibly negate the net catabolism of muscle in severely ill patients.
We previously found that enteral supplementation of glutamine alone was ineffective in replenishing glutamine availability in muscle and had no effect on muscle protein metabolism, as assessed using phenylalanine as an isotopic tracer.4 This previous study showed that despite a substantial increase in the arterial plasma concentration of glutamine and an increased flow of glutamine into the leg, the transport of glutamine into muscle was unaltered. This finding was in contrast to healthy volunteers in whom glutamine alimentation increased glutamine uptake within muscle, thereby reflecting a defect in glutamine transport into muscle of critically ill patients. These results fostered our pessimism for glutamine supplementation as a means to counteract muscle loss in catabolic patients.
Previous work in severely burned patients evidenced that coincident with the net increase in muscle protein catabolism and the depletion of muscle glutamine, there is a significant increase in the de novo production of alanine from the leg.20 Thus, this investigation suggested that after severe injury, glutamine production from muscle is reduced, whereas alanine production appears primarily responsible for nitrogen transport out of muscle. This present study also evidences an increased rate of de novo synthesis of alanine in conjunction with limitations in glutamine production from muscle, glutamine depletion, and a net catabolism of muscle protein in critically ill patients with prolonged sepsis. Because burn injury and sepsis are associated with accelerated glycolysis, this finding supports the relationship that increased pyruvate availability is a principal factor driving the increased production of alanine.21 This reciprocal relationship between alanine and glutamine production supports the notion that glutamate is preferentially deaminated in the formation of alanine, thereby potentially depleting precursor availability for glutamine synthesis. We thus theorized that glutamine production within muscle might be stimulated by supplementing enteral nutrition with a substantial amount of alanine, thereby increasing glutamate availability to support glutamine synthesis. We also postulated that valine may be of benefit by providing additional nitrogen for the production of glutamate from a-ketoglutarate and by augmenting availability of tricarboxylic acid cycle intermediates anaplerotically. The subsequent increase in [alpha]-ketoglutarate availability and possible reduction in nitrogen demand would thus diminish alanine efflux from muscle. Further, although from our previous study we knew that ingested glutamine would not be transported into muscle, we nonetheless supplemented glutamine with the thought that raising the plasma concentration would limit the rate of glutamine efflux from muscle.
This amino acid mixture of alanine, glutamine, and valine did significantly increase the respective arterial plasma concentrations. However, despite the increase in the inflow of alanine into muscle, inward transport (Fma) was not accelerated, and thus de novo synthesis was not affected. Similarly, neither glutamine transport nor the rate of de novo synthesis was affected by the greater influx of glutamine into the leg. Therefore, this amino acid mixture failed to affect the free glutamine concentration within muscle. These findings are consistent with our prior work indicating that the transport of glutamine into and out of muscle was unaffected by changes in glutamine availability in critically ill patients.4 Thus, the persistent net efflux of glutamine out of muscle appears to predominate the metabolic kinetics, thereby severely limiting the effectiveness of any dietary maneuvers to augment glutamine availability within muscle.
In contrast to the kinetic measurements of glutamine, the alimentation of this amino acid mixture and associated increase in alanine availability significantly decreased the net efflux of alanine from the leg. These findings suggest that alanine availability may influence the rate of alanine release from the muscle, yet kinetic measurements of alanine transport were not significantly altered.
Using phenylalanine to reflect muscle protein metabolism, this experiment failed to identify any influence of the alanine, glutamine, and valine amino acid mixture on muscle protein metabolism. A prior report by Hammarqvist et al22 suggested supplementation of total parenteral nutrition with alanyl-glutamine dipeptide may be beneficial in maintaining free glutamine concentrations in muscle and preserving muscle protein synthesis in patients undergoing cholecystectomy. This study used changes in ribosomal concentrations within muscle as a measure of muscle protein synthesis. A similar study using that methodology also demonstrated a beneficial effect of glutamine supplementation to augment muscle protein synthesis in postsurgical patients.3 However, recent work by Hammarqvist et al23 has failed to show any affect from glutamine supplementation alone on the rate of muscle protein synthesis when measured using the more direct method of fractional synthetic rate. Unfortunately, to date, these researchers have not repeated investigations with the alanyl-glutamine dipeptide using this more conventional methodology. One possibility to explain the different outcomes between the presented study and that performed by Hammarqvist et al22 is in the timing of alanine-glutamine supplementation. It is feasible that early institution of alanyl-glutamine may ameliorate the loss of glutamine from muscle and minimize limitations in muscle protein synthesis. Yet once glutamine is depleted from muscle, limitations in amino acid transport render subsequent supplementation of alanine, glutamine, and valine ineffective in either replenishing glutamine availability in muscle or influencing muscle protein metabolism. Another possible difference between the present study and that by Hammarqvist et al22 is the severity of illness of the study subjects. As in the work by Hammarqvist et al, those patients undergoing an elective cholecystectomy and receiving alanyl-glutamine maintained muscle free glutamine concentrations, whereas these values are severely reduced in the current study.
This failure of additional amino acid supplementation to influence muscle protein kinetics is consistent with that observed by Plank et al.24 Using serial analysis of body mass, these researchers found that variations in the quantity of protein intake did not affect the continued loss of lean body mass in critically ill, septic patients. This study by Plank et al,24 in conjunction with the presented work, suggests that dietary manipulation in the quality and above some minimal quantity of amino acid is unlikely to be of benefit in affecting the extent of muscle protein loss in critically ill patients. Because amino acid availability is a potent regulator of muscle protein synthesis in normal individuals,25 the failure of muscle in critically ill patients to respond to augmentation is amino acid availability reflects a fundamental defect. Thus, it is likely that some sort of metabolic manipulation such as hormonal (ie, insulin)26 or pharmacologic (ie, oxandrolone)27 will be needed to combat this defect.
ACKNOWLEDGMENTS
The work is supported by grants from Glucose Metabolism in Septic Patients (NIRO1DK33952) and Shrmers Grant: Special Shared Facility Mass Spectrometry (8490).
REFERENCES
1. Fong YM, Minei JP, Marano MA, et al: Skeletal muscle amino acid and myofibrillar protein mRNA response to thermal injury and infection. Am J Physiol 261:R536-R542, 1991
2. Rennie MJ, Hundal HS, Babij P, et al: Characteristics of a glutamine carrier in skeletal muscle have important consequences for nitrogen loss in injury, infection, and chronic disease. Lancet 2:1008-1012, 1986
3. Hammarqvist F, Wernerman J, Ali R, et al: Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg 209:455-461, 1989
4. Gore DC, Wolfe RR: Glutamine supplementation fails to affect muscle protein kinetics in critically ill patients. JPEN 26:342-350, 2002
5. Garber AJ, Karl IE, Kipnis DM: Alanine and glutamine synthesis and release from skeletal muscle: I. Glycolysis and amino acid release. J Biol Chem 251:826-835, 1976
6. Wolfe RR, Jahoor F, Hartl WH: Protein and amino acid metabolism after injury. Diabetes Metab Rev 5:149-164, 1989
7. Gore DC, Jahoor F, Hibbert J, et al: Except for alanine, muscle protein catabolism is not influenced by alterations in glucose metabolism during sepsis. Arch Surg 130:1171-1177, 1995
8. Bone RC: The sepsis syndrome: Definition and general approach to management. Clin Chest Med 17:175-181, 1996
9. Wahren J, Jorfeldt L: Determination of leg blood flow during exercise in man: An indicator-dilution technique based on femoral venous dye infusion. Clin Sci Mol Med Suppl 42:135-146, 1973
10. Biolo G, Fleming RY, Maggi SP, et al: Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol 268:E75-E84, 1995
11. Wolfe RR: Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. Wiley-Liss, New York, 1992
12. Ferrando AA, Lane HW, Stuart CA, et al: Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol 270:E627-E633, 1996
13. Biolo G, Gastaldelli A, Zhang XJ, et al: Protein synthesis and breakdown in skin and muscle: A leg model of amino acid kinetics. Am J Physiol 267:E467-E474, 1994
14. Biolo G, Chinkes D, Zhang XJ, et al: Harry M. Vars Research Award: A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN 16:305-315, 1992
15. Chinkes DL, Rosenblatt J, Wolfe RR: Assessment of the mathematical issues involved in measuring the fractional synthesis rate of protein using the flooding dose technique. Clin Sci (Lond) 84:177-183, 1993
16. Darmaun D: Role of glutamine depletion in severe illness. Diabetes Nutr Metab 13:25-30, 2000
17. Lacey JM, Wilmore DW: Is glutamine a conditionally essential amino acid? Nutr Rev 48:297-309, 1990
18. Boelens PG, Nijveldt RJ, Houdijk AP, et al: Glutamine alimentation in catabolic state. J Nutr 131:25698-25908, 2001
19. Wischmeyer PE, Lynch J, Liedel J, et al: Glutamine administration reduces gram-negative bacteremia in severely burned patients: A prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med 29:2075-2080, 2001
20. Biolo G, Maggi SP, Fleming RY, et al: Glutamine kinetics in skeletal muscle of severely burned patients: Transmembrane transport and intracellular de novo synthesis. JPEN 15:17S, 1991
21. Wolfe RR, Jahoor F, Herndon DN, et al: Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: Effect of dichloroacetate and glucose infusion. Surgery 110:54-67, 1991
22. Hammarqvist F, Wernerman J, von der Decken A, et al: Alanyl-glutamine counteracts the depletion of free glutamine and the postoperative decline in protein synthesis in skeletal muscle. Ann Surg 212:637-644, 1990
23. Hammarqvist F, Sandgren A, Andersson K, et al: Growth hormone together with glutamine-containing total parenteral nutrition maintains muscle glutamine levels and results in a less negative nitrogen balance after surgical trauma. Surgery 129:576-586, 2001
24. Plank LD, Connolly AB, Hill GL: Sequential changes in the metabolic response in severely septic patients during the first 23 days after the onset of peritonitis. Ann Surg 228:146-158, 1998
25. Rennie MJ, Bohe J, Wolfe RR: Latency, duration and dose response relationships of amino acid effects on human muscle protein synthesis. J Nutr 132:3225S-3227S, 2002
26. Ferrando AA, Chinkes DL, Wolf SE, et al: A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg 229:11-18, 1999
27. Sheffield-Moore M, Wolfe RR, Gore DC, et al: Combined effects of hyperaminoacidemia and oxandrolone on skeletal muscle protein synthesis. Am J Physiol Endocrinol Metab 278:E273-279, 2000
Dennis C. Gore, MD, and Robert R. Wolfe, PhD
From the Department of Surgery, The University of Texas Medical Branch, Galveston, Texas
Received for publication February 21, 2003.
Accepted for publication May 28, 2003.
Correspondence: Dennis C. Gore, MD, FACS, Associate Professor of Surgery, The University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555-1172.
Copyright American Society for Parenteral and Enteral Nutrition Sep/Oct 2003
Provided by ProQuest Information and Learning Company. All rights Reserved