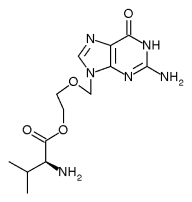
Valtrex[TM] (valacyclovir hydrochloride) has recently received expanded labeling indication by the U.S. Food and Drug Administration (FDA). Once daily 500 mg Valtrex Capleta have been approved for suppressive therapy in healthy adults with genital herpes in order to reduce the risk of transmission. It is estimated that 1 in 5 Americans are infected with the virus that causes genital herpes. However, as many as nine out of ten of those are unaware they have genital herpes, and clinical studies show that the majority of people with genital herpes contracted the disease from a partner who was otherwise asympmmatic.
In an eight month study of 1,484 healthy, heterosexual, monogamous couples, once daily suppressive therapy with Valtrex 500 mg caplets reduced the risk of transmission of symptomatic genital herpes by 75 percent versus placebo (0.5 percent on Valtrex vs. 2.2 percent on placebo). The primary, endpoint of the study was signs or symptoms of genital herpes to the uninfected partner. Reduced transmission was further con firmed with laboratory testing, and suppression therapy reduced rate of serologic conversion by 48 percent versus placebo (1.9 percent on Valtrex vs. 3.6 percent on placebo).
Valtrex is currently indicated for the treatment or suppression of genital herpes in healthy individuals and for the suppression of recurrent genital herpes in HIV-infected adults with CD4+ counts [greater than or equal to] 100 cells/uL. The most commonly reported side effects include headaches and upper respiratory symptoms. The effect of Valtrex on transmission of genital herpes has not been established in immunocompromised individuals or same-sex couples. The effect of Valtrex on reducing transmission beyond eight months has not been established.
Valtrex (valacyclovir hydrochloride) is manufactured and developed by GlaxoSmithKline.
COPYRIGHT 2003 Journal of Drugs in Dermatology
COPYRIGHT 2004 Gale Group