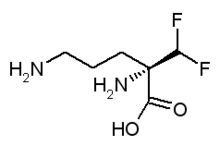
Barrier Therapeutics, Inc. (NASDAQ: BTRX), Princeton, N.J., a pharmaceutical company developing products in the field of dermatology, has acquired the right to distribute VANIQA(r) (eflornithine hydrochloride) Cream, 13.9% in Canada from Shire Pharmaceutical Contracts Limited. VANIQA(r) is currently the only prescription product approved by Health Canada for slowing the growth of unwanted facial hair in women.
Under the terms of the agreement, Barrier is the exclusive distributor for VANIQA(r) in the Canadian territory and is responsible for all sales, marketing, regulatory and distribution activities. Shire Pharmaceuticals is the supplier of finished goods for Barrier.
"Having acquired the rights to sell VANIQA(r) in Canada, along with our existing product Solag(r), we will immediately begin to build our own sales and marketing organization in Canada," commented Al Altomari, chief commercial officer. "We view Canada as an important market in our global commercialization plans, as it provides us an opportunity to leverage our existing commercial infrastructure."
About VANIQA(r) (eflornithine hydrochloride) Cream, 13.9% VANIQA(r) is the only prescription product approved by Health Canada for slowing the growth of unwanted facial hair in women. VANIQA(r) works during the growth phase of the hair cycle by blocking an enzyme that is necessary for hair growth. VANIQA(r) does not remove facial hair; instead, it is designed to reduce the rate of growth of facial hair and increase the interval between periods of hair removal when used together with traditional hair removal methods such as shaving, waxing and tweezing. Clinical trials have shown results from the use of VANIQA(r) in four to eight weeks from initial application, with continued improvement over time.
VANIQA(r) is approved for sale in 39 countries, including the United States, Canada, the European Union, and major Latin American countries.
About Barrier Therapeutics, Inc.
Barrier Therapeutics, Inc. is a pharmaceutical company focused on the discovery, development and commercialization of pharmaceutical products in the field of dermatology. The company currently markets Solag(r) (mequinol 2%, tretinoin 0.01%) Topical Solution in the U.S. and Canada for the treatment of solar lentigines, a common condition also known as "age spots" or "liver spots," and for the broader indication including related hyperpigmented lesions in Canada. Barrier has eight product candidates in various stages of clinical development. The four most advanced product candidates include one for the treatment of diaper dermatitis complicated by candidiasis, which is in registration, and three products, which are in or entering Phase 3 clinical trials for the treatment of seborrheic dermatitis, fungal infections, including vaginal candidiasis and onychomycosis, and congenital ichthyosis. Barrier has product candidates in earlier stages of clinical development for the treatment of acne, psoriasis and fungal infections. The company is headquartered in Princeton, New Jersey and has wholly owned subsidiaries in Geel, Belgium and Ontario, Canada.
For more information, call 212/845-4274 or visit http://www.barriertherapeutics.com.
COPYRIGHT 2005 Worldwide Videotex
COPYRIGHT 2005 Gale Group