Antiviral Therapy
In recent years, there has been increasing interest in the development of effective, relatively nontoxic antiviral agents. Foremost among these are the nucleoside analogs, synthetic compounds capable of interfering specifically with the synthesis of viral DNA and RNA. This article reviews currently available nucleoside analogs that are useful in the treatment of viral infections, as well as agents that are active against influenza A virus.
Acyclovir
MODE OF ACTION
The mechanism of action of acyclovir (Zovirax) has been extensively studied and serves as a prototype for that of the other nucleoside analogs. Acyclovir, an acyclic analog of guanosine, specifically inhibits replication of herpesviruses.[1] These viruses possess a thymidine kinase with a high degree of specificity for acyclovir; thus, cells expressing this viral thymidine kinase can selectively phosphorylate acyclovir to its monophosphate form. Cellular guanylate kinases catalyze the transformation of the monophosphate to acyclovir triphosphate. The triphosphate is a potent inhibitor of viral DNA polymerase, a necessary enzyme for viral genome replication. It also acts as an alternative substrate for viral DNA polymerase, promoting the incorporation of the monophosphate form into the DNA template. This results in irreversible damage to the viral genome.
Clinically, resistance to acyclovir has been rarely reported. It is generally an insignificant finding and is not clearly correlated with treatment failure.[2] In vitro mechanisms of resistance to the drug include (1) alterations in viral thymidine kinase that diminish enzyme activity or substrate specificity for acyclovir and (2) mutations that alter DNA polymerase, enhancing its ability to catalyze DNA replication despite the presence of high concentrations of acyclovir triphosphate. Only mutants with deficient or altered thymidine kinase activity have been reported clinically.
PHARMACOKINETICS
Acyclovir is slowly and incompletely absorbed in the gastrointestinal tract. Peak plasma concentrations occur one and one-half to two and one-half hours after oral administration. The bioavailability of acyclovir is between 15 and 30 percent and is not affected by food.
Acyclovir is widely distributed in tissues and body fluids, including the cerebrospinal fluid (CSF) and the aqueous humor. The drug persists in infected cells long after it has been cleared from the plasma. Approximately 14 percent of the administered dose is excreted unchanged in the urine. With normal creatinine clearance, the mean half-life of acyclovir is approximately three hours; in anuric patients, the half-life is extended to 19 and one-half hours. Since approximately 60 percent of the drug is removed with hemodialysis, patients should receive 60 to 100 percent of the loading dose after each dialysis.
APPROVED CLINICAL INDICATIONS
Acyclovir is approved for use in the treatment of infections caused by herpes simplex and varicella-zoster viruses. Although the drug possesses some in vitro activity against Epstein-Barr virus, there is no indication that it has any effect on the clinical course of infections caused by this virus. Acyclovir is not effective in the treatment of cytomegalovirus infections, because cytomegalovirus does not possess the thymidine kinase specific for the initial conversion of acyclovir to its monophosphate form.
At this time, acyclovir is available for topical, oral and intravenous administration. Topical therapy, with the 5 percent ointment or cream applied every four hours while the patient is awake, is sometimes useful in the treatment of genital and orolabial herpes simplex infections. A 3 percent ointment has been used in the treatment of herpes simplex keratitis. At this time, topical acyclovir therapy is not recommended for other uses.
The oral and intravenous forms of acyclovir are more clinically useful. Both oral acyclovir, 200 mg every four hours while the patient is awake, or five times daily, and intravenous acyclovir, 5 mg per kg every eight hours, are effective in reducing symptoms, viral shedding and healing time in primary genital herpes. Oral acyclovir also decreases the duration of viral shedding and new lesion formation in recurrent herpes genitalis.[3] When administered orally in a dosage of 200 mg twice daily, acyclovir suppresses recurrence of genital herpes in 60 to 90 percent of patients.[2]
Acyclovir therapy has also been effective in other herpes simplex infections, including neonatal infections, keratitis, acute necrotizing retinitis, and visceral and anorectal infections in the immunocompromised host. The oral and intravenous forms of acyclovir successfully prevent recurrence of visceral or mucocutaneous herpes simplex in bone marrow and renal transplantation patients. At higher doses (10 mg per kg every eight hours), intravenous acyclovir is the treatment of choice for herpes simplex encephalitis.[4]
Acyclovir is also effective in the treatment of varicella-zoster and herpes zoster infections in the immunocompromised host,[5] and it may speed recovery in herpes zoster encephalitis.[6] Although acyclovir decreases viral shedding, the duration of skin rash and the incidence of episcleritis, keratitis and iritis in the normal host, it has no effect on postherpetic neuralgia. Because varicella-zoster virus is relatively more resistant to acyclovir than is herpes simplex virus, higher doses (10 mg per kg intravenously every eight hours or 800 mg orally every four hours while the patient is awake) should be used for the treatment of zoster-related infections.
ADVERSE EFFECTS
Acyclovir is generally well tolerated, and significant side effects occur infrequently. High-dose intravenous administration has been associated with local irritation and phlebitis. Topical therapy also may cause local irritation. Allergic reactions are theoretically possible, but none have been reported; however, acute administration has been associated with nausea, hypotension, rash and rigors.
The toxicity of greatest concern is reversible azotemia secondary to the crystallization of acyclovir in the rental tubules or collecting ducts. Like the other adverse effects associated with acyclovir, azotemia appears to be dose-related, and its incidence is increased with dehydration, preexisting renal insufficiency and bolus infusion.[7]
Vidarabine
MODE OF ACTION
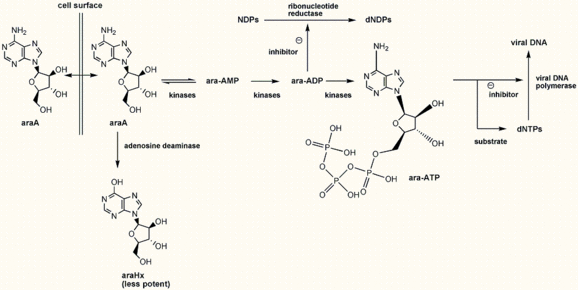
Vidarabine (Vira-A) is an adenine deoxyriboside analog capable of inhibiting viral DNA synthesis by a mechanism similar to that of the other nucleoside analogs. Unlike acyclovir, vidarabine does not require a viral thymidine kinase for phosphorylation, but instead uses cellular enzymes for conversion to the triphosphate form.[8] The triphosphate inhibits the action of both viral and cellular DNA polymerases, but it appears to have greater specificity for the viral polymerase. In addition, the triphosphate is presumed to act as a chain terminator in DNA synthesis by becoming incorporated into viral DNA. Vidarabine triphosphate may also inhibit virus-induced ribonucleotide reductase.
PHARMACOKINETICS
Vidarabine is poorly soluble and therefore requires large fluid volumes for intravenous administration, the primary mode of delivery. Following intravenous administration, vidarabine is rapidly converted to its less active metabolite, arabinosyl hypoxanthine, by adenosine deaminase, an enzyme present in serum and red blood cells, as well as throughout the body. Arabinosyl hypoxanthine is widely distributed and has been detected in the CSF. Since excretion of vidarabine is primarily renal, the dosage should be reduced in patients with impaired renal function.
APPROVED CLINICAL INDICATIONS
Intravenous vidarabine is effective in the treatment of herpes simplex infections, particularly encephalitis (15 mg per kg per day)[9] and neonatal disease,[10] and for the treatment of varicella or herpes zoster in the immunocompromised host (10 mg per kg per day).[11] A topical form of vidarabine is available, but there is no oral preparation. Topical vidarabine (3 percent ointment) is efficacious in the treatment of acute and recurrent herpes keratitis.
Although vidarabine has in vitro activity against cytomegalovirus, pox viruses, Epstein-Barr virus, adenoviruses and hepatitis B virus, there have been no controlled studies using vidarabine for infections caused by any of these agents. In fact, the less toxic and much more effective acyclovir has supplanted vidarabine in the treatment of most herpes virus infections.
ADVERSE EFFECTS
Vidarabine has been associated with many significant toxic side effects, particularly when it is administered in higher doses or when it is given to patients with renal dysfunction or to patients receiving concurrent interferon therapy. The most common adverse effects are gastrointestinal, particularly anorexia, nausea, vomiting and diarrhea. Reversible anemia, leukopenia, thrombocytopenia, rashes and thrombophlebitis have also been noted.
The neurologic toxicities associated with vidarabine are of special concern. These have included tremors, pain syndromes, seizures, alterations in mental status, ataxia, motor aphasia, dysarthria and death.
Ribavirin
MODE OF ACTION
A pyrimidine nucleoside analog, ribavirin (Virazole) depends on the host cell enzyme, adenosine kinase, for phosphorylation to the triphosphate form. Consequently, ribavirin causes general inhibition of RNA synthesis, specifically the inhibition of viral RNA and protein synthesis as well as the host cell messenger RNA synthesis necessary for terminal capping and subsequent priming of some viral messenger RNAs.[12]
PHARMACOKINETICS
Aerosol administration, the primary mode of delivery, is associated with minimal systemic absorption. After oral or intravenous administration, ribavirin has been found in multiple sites, including the CSF. Elimination occurs rapidly, primarily through renal excretion.
APPROVED CLINICAL INDICATIONS
Although ribavirin has in vitro activity against many DNA and RNA viruses, clinical use of the drug has been limited to the treatment of respiratory syncytial virus infections (estimated dose of aerosolized form: 0.8 mg per kg per hour of administration)[13] and Lassa fever (oral or intravenous form).[14] Aerosolized ribavirin may also be effective in the treatment of some influenza virus strains.[15] At this time, however, ribavirin is licensed for use in the United States only for the treatment of respiratory syncytial virus infections in hospitalized infants and young children.
ADVERSE EFFECTS
In general, aerosolized ribavirin is extremely well tolerated, with only rare reports of deterioration of pulmonary function. Oral ribavirin therapy has been associated with reversible anemia and transient elevations of serum bilirubin.
Zidovudine
MODE OF ACTION
Zidovudine (AZT; Retrovir) is a thymidine analog that is phosphorylated to the triphosphate form by cellular enzymes.[16] Zidovudine triphosphate interferes with the viral RNA-dependent DNA polymerase (reverse transcriptase), thereby terminating DNA chain elongation. In addition, zidovudine decreases thymidine triphosphate synthesis by competitively inhibiting thymidylate kinase. This reduces the availability of the cellular substrate for RNA-dependent DNA polymerase, consequently enhancing the binding of zidovudine triphosphate to the polymerase.
PHARMACOKINETICS
Zidovudine is only available commercially as an oral medication. It is well absorbed and appears to be widely distributed throughout the body, including the CSF. Elimination involves both hepatic glucuronidation and urinary excretion.
APPROVED CLINICAL INDICATIONS
In vitro, zidovudine is active against human retroviruses (including the human immunodeficiency virus [HIV]), Epstein-Barr virus and some bacteria. The only currently approved indication for use is in patients with symptomatic HIV infection (acquired immunodeficiency syndrome [AIDS] or advanced AIDS-related complex [ARC]), particularly those with a history of Pneumocystis carinii pneumonia or markedly diminished T-helper/inducer lymphocyte counts (CD4 less than 200 per [mm.sup.3]). When treated with zidovudine, these patients have demonstrated increased CD4 counts, some restoration of immunologic function, a significant decrease in the risk and severity of opportunistic infections, and a decrease in mortality.[17] The effects of zidovudine therapy are temporary, and their duration appears to vary from patient to patient. The current dosage recommendation is 200 mg every four hours.
ADVERSE REACTIONS
Unfortunately, zidovudine has been associated with significant adverse reactions that limit its use.[18] Foremost among these are macrocytic anemia and granulocytopenia, both noted most frequently in patients with depressed CD4 counts, anemia, neutropenia or vitamin [B.sub.12] deficiency prior to therapy. Central nervous system toxicity (especially headaches and confusion), gastrointestinal disturbances (primarily nausea), fever, rash and increased nail pigmentation have also been noted.
A number of drugs, including pentamidine (Pentam), dapsone, amphotericin B (Fungizone), flucytosine (Ancobon), doxorubicin (Adriamycin) and interferon, may increase the toxic effects of zidovudine. Aspirin, acetaminophen or indomethacin (Indocin) may inhibit glucuronidation of zidovudine. Coadministration of zidovudine with any of these agents may increase the risk of toxicity.
Idoxuridine
Idoxuridine (Herplex, Stoxil) is an iodinated analog of thymidine capable of inhibiting DNA synthesis in both viral and host cells. Because of toxicity associated with systemic administration and the general lack of clinical efficacy, the use of idoxuridine has been restricted to topical therapy for herpes simplex keratitis. Topical application has been associated with local inflammatory and allergic responses.
Ganciclovir
Ganciclovir is an experimental acyclic nucleoside currently under investigation for the treatment of serious cytomegalovirus infections in patients with AIDS and in recipients of organ transplants (especially bone marrow). Like the previously mentioned acyclic nucleosides, ganciclovir is converted to the active triphosphate form by cellular enzymes. The triphosphate selectively inhibits the DNA polymerase necessary for virus replication.[19]
Clinically, ganciclovir has been used with varying degrees of success in the treatment of cytomegalovirus infections.[20] Its greatest efficacy has been demonstrated in cases of retinitis.[21] Recent studies suggest that gastrointestinal disease also may respond to ganciclovir therapy.[22] Relapses are common when the drug is withdrawn; thus, long-term maintenance therapy appears to be necessary to ensure a disease-free interval.
Since ganciclovir can be incorporated into host cell DNA, it has significant potential for toxicity. The most serious side effect has been leukopenia.
Amantadine and Rimantadine
MODE OF ACTION
Amantadine (Symmetrel) and rimantadine (Flumadine) are structurally similar amines with specific activity against influenza A virus. The antiviral action of amantadine against influenza A virus is not completely understood. The mechanism of action appears to involve interference with replication, probably by blocking a late stage of viral uncoating.
PHARMACOKINETICS
Amantadine and rimantadine are available as oral preparations. Amantadine is excreted by the kidney without being metabolized. Rimantadine also undergoes urinary excretion, but it is largely metabolized before elimination. Both medications require dose reductions in elderly patients and in patients with renal failure.
APPROVED CLINICAL INDICATIONS
At this time, only amantadine is available for use in the United States. In addition to its use in Parkinson's disease, amantadine is indicated for influenza A prophylaxis in patients who receive late influenza vaccination, in unvaccinated close contacts of patients at high risk for influenza A, in immunodeficient patients who may have a poor response to vaccination, and in patients for whom influenza vaccine is contraindicated.[23]
Prophylaxis is only effective during the period in which amantadine is being administered. The usual dosage in adults is 100 mg twice daily, but 100 mg per day may be as effective and may induce fewer side effects. Prophylaxis should be given for the duration of influenza activity in the community in those patients who are not immunized.
Amantadine appears to reduce the severity and diminish the duration of influenza A illness in healthy adults and children. Thus, amantadine therapy should be considered if the drug can be given within 24 to 48 hours of the onset of influenza symptoms.
ADVERSE EFFECTS
Amantadine toxicity appears to be dose-related, and it occurs most often in the elderly and in patients with renal failure. The most significant adverse effects are neurologic, particularly confusion, dizziness, nervousness and insomnia. Rashes, seizures, postural hypotension and leukopenia have also been reported.
Rimantadine appears to be better tolerated than amantadine. With similar doses of the two medications, serum levels of rimantadine are lower than those of amantadine. At comparable serum levels, similar toxicities have been noted.[24]
Final Comment
Antiviral agents are now available in safe and effective forms for the treatment of several viral infections, especially those caused by the herpesviruses. The development of new antiviral medications continues to be a focus of research. In the near future, we can expect to see an increasing number of agents that are active against a greater number of disease-causing viruses.
REFERENCES [1]Dorsky DI, Crumpacker CS. Drugs five years later: acyclovir. Ann Intern Med 1987;107:859-74. [2]Lehrman SN, Douglas JM, Corey L, Barry DW. Recurrent genital herpes and suppressive oral acyclovir therapy. Relation between clinical outcome and in-vitro drug sensitivity. Ann Intern Med 1986;104:786-90. [3]Nilsen AE, Aasen T, Halsos AM, et al. Efficacy of oral acyclovir in the treatment of initial and recurrent genital herpes. Lancet 1982;2(8298):571-3. [4]Whitley RJ, Alford CA, Hirsch MS, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med 1986;314:144-9. [5]Shepp DH, Dandliker PS, Meyers JD. Treatment of varicella-zoster virus infection in severely immunocompromised patients. A randomized comparison of acyclovir and vidarabine. N Engl J Med 1986;314:208-12. [6]Johns DR, Gress DR. Rapid response to acyclovir in herpes zoster-associated encephalitis. Am J Med 1987;82:560-2. [7]Bean B, Aeppli D. Adverse effects of high-dose intravenous acyclovir in ambulatory patients with acute herpes zoster. J Infect Dis 1985;151:362-5. [8]De Clercq E, Descamps J, Verhelst G, et al. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J Infect Dis 1980;141:563-74. [9]Whitley RJ, Soong SJ, Dolin R, Galasso GJ, Ch-ien LT, Alford CA. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious Diseases collaborative antiviral study. N Engl J Med 1977;297:289-94. [10]Whitley RJ, Nahmias AJ, Soong SJ, Galasso GG, Fleming CL, Alford CA. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics 1980;66:495-501. [11]Whitley RJ, Soong SJ, Dolin R, Betts R, Linnemann C Jr, Alford CA Jr. Early vidarabine therapy to control the complications of herpes zoster in immunosuppressed patients. N Engl J Med 1982;307:971-5. [12]Browne MJ. Mechanism and specificity of action of ribavirin. Antimicrob Agents Chemother 1979;15:747-53. [13]Hall CB, McBride JT, Walsh EE, et al. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. A randomized double-blind study. N Engl J Med 1983;308:1443-7. [14]McCormick JB, King IJ, Webb PA, et al. Lassa fever. Effective therapy with ribavirin. N Engl J Med 1986;314:20-6. [15]Gilbert BE, Wilson SZ, Knight V, et al. Ribavirin small-particle aerosol treatment of infections caused by influenza virus strains A/Victoria/7/83 (H1N1) and B/Texas/1/84. Antimicrob Agents Chemother 1985;27:309-13. [16]Hirsch MS. AIDS commentary. Azidothymidine. J Infect Dis 1988;157:427-31. [17]Fischl MA, Richman DD, Grieco MH, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med 1987;317:185-91. [18]Richman DD, Fischl MA, Grieco MH, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med 1987;317:192-7. [19]Matthews T, Boehme R. Antiviral activity and mechanism of action of ganciclovir. Rev Infect Dis 1988;10(Suppl 3):S490-4. [20]Collaborative DHPG Treatment Study Group. Treatment of serious cytomegalovirus infections with 9-(1,3-dihydroxy-2-propoxymethyl) guanine in patients with AIDS and other immunodeficiencies. N Engl J Med 1986;314:801-5. [21]Rosecan LR, Stahl-Bayliss CM, Kalman CM, Laskin OL. Antiviral therapy for cytomegalovirus retinitis in AIDS with dihydroxy propoxymethyl guanine. Am J Ophthalmol 1986;101:405-18. [22]Chachoua A, Dieterich D, Krasinski K, et al. 9-(1,3-dihydroxy-2-propoxymethyl) guanine (ganciclovir) in the treatment of cytomegalovirus gastrointestinal disease with the acquired immunodeficiency syndrome. Ann Intern Med 1987;107:133-7. [23]Prevention and control of influenza. MMWR 1988;37:361-4,369-73. [24]Hayden FG, Hoffman HE, Spyker DA. Differences in side effects of amantadine hydrochloride and rimantadine hydrochloride relate to differences in pharmacokinetics. Antimicrob Agents Chemother 1983;23:458-64.
EMILY A. BLUMBERG, M.D., Assistant Professor of Medicine Hahnemann University School of Medicine, Philadelphia, Pennsylvania JOSEPH R. DIPALMA, M.D., coordinator of this series, is emeritus professor of pharmacology and medicine at Hannemann University School of Medicine, Philadelphia.
COPYRIGHT 1989 American Academy of Family Physicians
COPYRIGHT 2004 Gale Group