Videx
Didanosine (2'-3'-dideoxyinosine, ddI) is sold uner the trade names Videx® and Videx EC®. It is a reverse transcriptase inhibitor, effective against HIV and usually used in combination with other antiviral drug therapy as part of highly active antiretroviral therapy (HAART). more...
History
Didanosine was developed by Samuel Broder, Hiroaki Mitsuya, and Robert Yarchoan in the National Cancer Institute (NCI). Since the NCI cannot market a product, the National Institutes of Health (NIH) awarded a ten-year exclusive licensed to Bristol-Myers Squibb Co. (BMS) to market and sell ddI as Videx® tablets.
Didanosine became the second drug approved for the treatment of HIV infection in many other countries, including in the United States by the Food and Drug Administration (FDA) on Oct 9, 1991. Its FDA approval helped bring down the price of zidovudine (AZT), the initial anti-HIV drug.
Didanosine has weak acid stability and is easily damaged by stomach acid. Therefore, the original formula approved by the FDA used chewable tablets that included an antacid buffering compound to neutralize stomach acid. The chewable tablets were not only large and fragile, they also were foul-tasting and the buffering compound would cause diarrhea. Although the FDA had not approved the original formulation for once-a-day dosing it was possible for some people to take it that way.
At the end of its ten-year license, BMS re-formulated Videx® as Videx EC® and patented that, which reformulation the FDA approved in 2000. The new formulation is a smaller capsule containing coated microspheres instead of using a buffering compound. It is approved by the FDA for once-a-day dosing. Also at the end of that ten-year period, the NIH licensed didanosine to Barr Laboratories under a non-exclusive license, and didanosine became the first generic anti-HIV drug marketed in the United States.
One of the patents for ddI will expire in the United States on 2006-08-29, but other patents extend beyond that time.
Mechanism of action
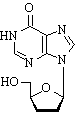
Didanosine (ddI) is a nucleoside analogue of adenosine. It differs from other nucleoside analogues, because it does not have any of the regular bases, instead it has hypoxanthine attached to the sugar ring. Within the cell, ddI is, by cellular enzymes, phosphorylated to active metabolite of dideoxyadenosine triphosphate, ddATP. Like other anti-HIV nucleside analogs, it acts as a chain terminator by incorporation and inhibits viral reverse transcriptase by competing with natural dATP.
Oral absorption of ddI is fairly low (40%) but rapid. The half-life in plasma is only 30 minutes, but in intracellular environment more than 12 hours. An enteric-coated formulation is now marketed as well. The kidneys actively secrete didanosine, the amount being 20 % of the oral dose.
Adverse affects
The side effects of didanosine are mainly headache and nausea, but also peripheral neuropathy, insomnia, pancreatitis and alterations of liver functions have been reported. Drug resistance to didanosine does develop, though slower than to Zidovudine (AZT).
Read more at Wikipedia.org