Abstract
The stability of a 2-mg/mL zidovudine and 0.5-mg/mL ranitidine admixture stored in 0.9% sodium chloride and 5% dextrose injections in 50-mL polyvinylchloride bags at ambient temperature and 4°C up to 24 hours was studied. The samples were analyzed at 0, 4, 8 and 24 hours after preparation of the controls and admixtures by means of a reverse-phase, stability-indicating, high-performance liquid Chromatographic method. The drug mixtures were stable for up to 24 hours, showing levels of greater than 90% of the initial drug concentrations at the time of preparation. The pH of the admixtures was in the pH 5-6 range. These data support the stability of the zidovudine and ranitidine admixture under the storage conditions studied.
Introduction
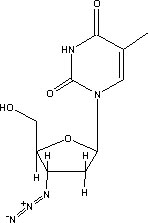
Zidovudine (3'-azido-3'-dcoxythymidine), popularly known as AZT, is a nucleoside analogue approved by the US Food and Drug Administration in 1987 for the treatment of AIDS.1 Zidovudine differs from thymidine in having an azido (N^sub 3^) group at the 3' position of the deoxyribose ring (Figure 1). Zidovudine is stable in 5% dextrose injection and 0.9% sodium chloride injection up to 24 hours at ambient temperature (23 ± 2°C) and up to 48 hours at 2° to 8°C.2 Ranitidine hydrochloride (Figure 2) is an H^sub 2^ receptor agonist widely used for the treatment of gastric and duodenal ulcers, Zollinger Ellison syndrome, reflux esophagitis, dyspepsia and other hypersecretory conditions.3-4 Ranitidine has been shown to be stable in various admixtures under ambient, refrigerated and frozen storage conditions.5-6 The stability of zidovudine and ranitidine admixtures has not been reported. The purpose of this study was to investigate the stability of 2-mg/mL zidovudine and 0.5-mg/inL ranitidine as an admixture prepared in polyvinylchloride (PVC) bags stored at either ambient temperature or 4°C for up to 24 hours. Each bag was assayed for drug concentration and the pH was measured at 0, 4, 8 and 24 hours after the bags had been prepared.
Methods
Chemicals and Reagents
All chemicals (J.T. Baker, Phillipsburg, New Jersey) were high-performance liquid Chromatographie (HPLC) grade. Zidovudine and Ranitidinc Hydrochloride reference standards were obtained from the United States Pharmacopcial Convention, Inc. (Rockville, Maryland). Zidovudine infusion 10-mg/mL vials and ranitidine hydrochloridc 25-mg/mL vials were obtained from GlaxoSmithKline (Research Triangle Park, North Carolina). The 0.9% sodium chloride injection and S% dextrose injection in 50-mL PVC hags were obtained from Cardinal Health, (McDonough, Georgia). Sodium dihydrogen phosphate was obtained from Fischer Scientific (Pittsburgh, Pennsylvania). HPLC-grade deionixcd water was obtained by use of cartridges from Continental Water System (Roswell, Georgia).
Equipment
The HPLC system consisted of a Beckman Model 110 solvent delivery system pump (Fullerton, California), a Rheodyne injector (Model 7125, Rheodyne, Cotati, California) equipped with a 20-µL loop and a lambda max ultraviolet (UV) detector Model 481 (Waters Corporation, Milford, Massachusetts) set at 265 nm. The peak heights were monitored with a Shimadzu Model CR3A electronic integrator (Columbia, Maryland). A locally built microprocessor-controlled pH meter with autocalibration and accuracy to 0.01 pH units, a C^sub 18^ column that contained an amide hexadecylsilane packing material (4.6 mm × 250 mm, 5-µm particle size, Supelco, Bellefonte, Pennsylvania), Nylon-66 membrane filters (0.2 µm × 47 mm, Supelco) and a 60-cc syringe (Tyco Health Systems, Mansfield, Massachusetts) were used.
Chromatographic Conditions
The isocratic elutions were performed at 1 mL/min with a mobile phase consisting of 12:88 v/v acetonitrile-phosphate buffer (25 mM) adjusted to pH 3.0. The mobile phase was filtered through a 0.2-µm nylon filter prior to use.
Preparation of Polyvinylchloride Bags for Stability Studies
A pooled sample of 200 mL containing 2-mg/mL zidovudine and 0.5-mg/mL ranitidine hydrochloride admixture was prepared by adding 0.9% sodium chloride injection to a suitably sized beaker. After thorough mixing, 5 mL of the solution was removed for zero-hour assay and the remaining 195 mL was evenly divided into six empty 50-mL PVC bags.
A second pooled sample of 200 containing 2-mg/mL zidovudine and 0.5-mg/mL ranitidine hydrochloride was prepared by adding 5% dextrose injection to a suitably sized beaker. After thorough mixing, 5 mL of the solution was removed for timezero hour assay and the remaining 195 mL was evenly divided into six empty 50-mL PVC bags. Appropriate controls of zidovudine and ranitidine in 0.9% sodium chloride and 5% dextrose injections were also prepared.
Three bags from each pooled sample were stored at 23 ± 2°C under continuous fluorescent light and three bags were stored under refrigeration at 4°C. The drug content in each bag was determined by a stability-indicating HPLC assay at 0, 4, 8 and 24 hours, and the pH values were also recorded at those times. The controls of zidovudine and ranitidine stored at ambient temperature and 4°C were also assayed, and pH values recorded.
Preparation of Standard Solutions
Two 2-mg weighed quantities of zidovudine USP reference standard were diluted with 1 mL of 0.9% sodium chloride injection and 1 mL of 5% dextrose injection to give two solutions, each containing 2 mg/mL. The solutions were further diluted 1:80 to give final concentrations of 25 µg/mL. Diluted standards of 100 µL were injected in triplicate into the HPLC system to calculate the individual response factor (RF).
Two 1.0-mg weighed quantities of ranitidine USP reference standard were diluted with 1 mL of 0.9% sodium chloride injection and 1 mL of 5% dextrose injection to give solutions each containing 1 mg/mL. The solutions were further diluted 1:80 to give concentrations of 12.5 µg/mL. Of the diluted standard, 100 µL was injected in triplicate onto the HPLC to calculate the RF. All standard solutions were prepared fresh daily.
Degradation of Zidovudine and Ranitidine
To ensure that the HPLC assay was stability indicating, zidovudine and ranitidine were previously forced to degrade under acidic, basic, oxidative and UV irradiation conditions. The method was developed and validated in our labs prior to this study.7
Preparation of Assay Solutions
A 5-mL aliquot was removed from each PVC bag stored at ambient temperature and 4°C at 0, 4, 8 and 24 hours. A 1:80 dilution was made using the mobile phase as a diluent, and 100 µL of the diluted sample was injected in triplicate into the HPLC system.
Calculation of Medication Content
Triplicate injections of both the analytical samples prepared from PVC bags and the standard solutions were injected into the HPLC system. Peak heights from the ehromatogram were used to calculate the mean response factor (MRF) for each drug standard. The appropriate MRF and the peak height of each analyte in the PVC bag and standard samples were used to calculate the drug concentration in each analytical sample. The following calculations were used to determine the drug concentration in each sample.
Response factor (RF) = Drug standard (mg/mL)/Drug peak height of standard
Calculate the MRF by using three replicates of each standard
Drug concentration (mg/mL) = MRF × Drug peak height of the sample
Results
A typical FIPLC ehromatogram of the zidovudine and ranitidine mixture is shown in Figure 3. The zidovudine and ranitidine admixtures prepared in 0.9% sodium chloride injection or 5% dextrose injection stored in 50-mL PVC bags were judged to be stable if the drug levels of the remaining initial drug were greater than 90% of the concentration at the time of preparation.8 The mean percent remaining data for zidovudine and ranitidine are shown in Tables 1 through 4. The pH of the admixtures ranged from 5.07 to 5.90.
Conclusion
Zidovudine (2-mg/mL) and ranitidinc (0.5-mg/mL) injections stored as admixtures in 50-mL PVC bags were stable for 24 hours at 23 ± 2°C and 4°C in both 0.9% sodium chloride injection and 5% dextrose injection. Pharmacists should note that admixtures in dextrose injections should not be stored for longer than 24 hours at ambient temperature.
References
1. Zimmerman TP, M a honey WB, Prus KL. 2'-3' Azidothymidine. J Biol Chem 1986; 10:5748-5754.
2. Lam NP, Kennedy PE, Jarosinski PF et al. Stability of zidovudine in 5% dextrose injection and 0.9% sodium chloride injection. Am J Hasp Pharm 1991; 48(2): 281-282.
3. Brogden RN, Carmine AA, Heel RC et al. Ranitidine: A review of its pharmacology and therapeutic use in peptic ulcer disease and other allied diseases. Drugs 1982; 24(4): 267-303.
4. Saunders JB, Oliver RJ, Darral LH. Treatment of esophageal reflux disease. British Medical Journal WW; 282: 86-87.
5. Lampasona V, Mullins RE, Parks RB. Stability of ranitidine admixtures frozen and refrigerated in minibags. Am J Hosp Pharm 1986; 43(4): 921-925.
6. Stewart JT, Warren FW, King AD. Stability of ranitidine hydrochloride and seven medications. Am J Hosp Pharm 1994; 51(14): 1802-1807.
7. Caufield WV, Stewart JT. HPLC separations of zidovudine and selected pharmaceuticals using a hexadecylsilane amide column. Chromatographie 2001; 54(9): 561-568.
8. Trissel LA. Avoiding common flaws in stability and compatibility studies of injectable drugs. Am J Hosp Pharm 1983; 40(7): 1159-1160.
Patrick Musami, MS
James T. Stewart, PhD
E. Will Taylor, PhD
Department of Pharmaceutical and Biomedical Sciences
College of Pharmacy
University of Georgia
Athens, Georgia
Address correspondence to: James T. Stewart, PhD, Department of Pharmaceutical and Biomedical Sciences, College of Pharmacy, University of Georgia, Athens, GA 30606. E-mail: jstewart@mail.rx.uga.edu
Copyright International Journal of Pharmaceutical Compounding May/Jun 2004
Provided by ProQuest Information and Learning Company. All rights Reserved