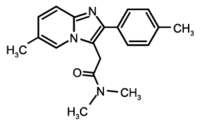
Zolpidem is a hypnotic drug of the imidazopyridine group. Another imidazopyridine, alpidem, has been withdrawn from the market because of its hepatotoxicity.[1 2] Hepatotoxicity has been suspected in association with zolpidem, but it has not been clearly established because of concomitant drug treatment.[3 4] We report a case of acute hepatitis mimicking biliary lithiasis after treatment with zolpidem alone at a therapeutic dose, with reappearance of the hepatoxicity after the drug was reintroduced.
A 53 year old woman was admitted in June 1997 for investigation of recurrent abdominal pain. She had no history of recent travel, drug addiction, blood transfusion, or chronic intake of alcohol or toxins. She had first taken zolpidem for insomnia in July 1996. She had had a cholecystectomy for cholangitis in the same month, but we could not clearly establish a chronological link between zolpidem ingestion and this acute episode.
In September 1996 she had again taken zolpidem (20 mg at bed time), and two days later she had developed sudden epigastric pain associated with pale stools, dark urine, but no fever. She had then decided to stop taking zolpidem. The abdominal pain had spontaneously disappeared within 12 hours. Biological investigations performed four days later, when jaundice had been regressing, had shown serum activities of alanine aminotransferase to be 596 IU/l (normal range 5-31), aspartate aminotransferase to be 198 IU/l (8-31), [Gamma]-glutamyl transpeptidase to be 242 IU/l (5-35), and alkaline phosphatase to be 134 IU/l (30-104). Total blood bilirubin concentration had been 21.2 [micro]mol/l and the prothrombin time had been normal. Eight days later serum activities of alanine aminotransferase and [Gamma]-glutamyl transpeptidase had been 95 IU/l and 115 IU/l respectively. Retrograde endoscopic cholangiography had shown no abnormality.
Six months later, in April 1997, she had had another episode of abdominal pain. Eleven days later alanine aminotransferase and [Gamma]-glutamyl transpeptidase activities had been 50 IU/l and 89 IU/l respectively.
In June 1997 ultrasound examination of the biliary tract gave normal results. On questioning she remembered that zolpidem had been reintroduced because her insomnia had recurred (she had taken 20 mg two days before the last acute episode). Viral hepatitis and concurrent infections with Epstein-Barr virus and cytomegalovirus were excluded. No antibodies against smooth muscle, liver and kidney microsomes, or liver cytosol or mitochondria were detected in serum. Five months later the results of liver function tests remained within normal limits and she had no symptoms.
A causal association between zolpidem treatment and liver damage is likely in our case because of the time of onset of the reaction, the clinically significant decrease in serum alanine aminotransferase activity, the exclusion of other causes of hepatitis, the presence of a normal biliary tract on ultrasound and radiological examination, and, above all, the recurrence with zolpidem readministration.[5]
To date, our pharmacovigilance service has not been informed of any other cases of hepatoxicity associated with zolpidem given alone at a therapeutic dose.
[1] Ausset P, Malavialle P, Vallet A, Minemont G, Le Bail B, Dumas F, et al. Hepatite subfulminante a l'alpidem traitee par transplantation hepatique. Gastroenterologie Clinique et Biologique 1995; 19:222-35.
[2] Baty V. Hepatite imputable a l'alpidem (Ananxyl), quatre cas dont un mortel. Gastroenterologie Clinique et Biologique 1994; 18:1129-31.
[3] Queneau PE, Koch S, Hrusovsky S, Miguet JP. Zolpidem. Cytolytic hepatitis related to zolpidem. First international symposium on hepatology and clinical pharmacology liver and drugs 1994;38. (abstract). [Available on Medline.]
[4] Garnier R. Acute zolpidem poisoning--analysis of 344 cases. Clin Toxicol 1994;32:391-404.
[5] Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 1990;11:272-6.
David Karsenti, Pascal Blanc, Yannick Bacq, Etienne-Henry Metman, Service d'Hepato-Gastroenterologie, Centre Hospitalier Universitaire Trousseau, and Service de Pharmacologie Clinique, Centre Hospitalier Universitaire Bretonneau, F-37044 Tours Cedex, France
COPYRIGHT 1999 British Medical Association
COPYRIGHT 2000 Gale Group