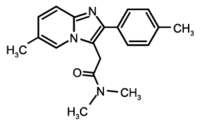
Study objective: To assess the effects of repeated 10-mg oral doses of zolpidem on diurnal and nocturnal respiratory function, as well as on diurnal vigilance and physical performance in COPD patients with disordered sleep. Design: Prospective single-blind placebo-controlled clinical study. Setting: Outpatients of a respiratory medicine department. Patients and methods: Patients with stable COPD were enrolled for 10 days (D0 to D10), ie, 9 consecutive nights (N1 to N9). They received placebo on N1 and N9 and zolpidem, 10 mg, from N2 to N8. Measurements: The following parameters were measured: nocturnal polysomnographic recordings with respiratory signals and arterial blood gas values on retiring and awakening on N0, N1, N2, N8, and N9; subjective evaluation of the quality of sleep and of diurnal vigilance by visual analog scales every day from D0 to D10; pulmonary function test, central control of breathing, and walking test on D0 and D9; biological laboratory tests and theophylline level on D0 and D8. Results: Ten COPD patients (Pa[O.sub.2]=72.7 [+ or -] 7.6 mm Hg; PaC[O.sub.2]=47.7 [+ or -] 5.4 mm Hg; FEV1=0.84 [+ or -] 0.3 L; FE[V.sub.1]/vital capacity=42.5 [+ or -] 12.3%), 56.8 [+ or -] 8.3 years old, were studied. Compared with placebo, no significant change was found for the various sleep architecture parameters, except an increase in the duration of stage 2 during the D8/N8 night (p<0.05). In contrast, the autoevaluation score for the quality of sleep was significantly improved during the D6/N6 night relative to that with placebo (p<0.05), with no change in the other subjective criteria. No variable of the nocturnal respiratory parameters, pulmonary function test, central control of breathing, and physical performance was altered by zolpidem. Arterial blood gas values on awakening were not altered. Clinical and biological tolerance of zolpidem was correct with no significant variation of the theophylline level. Conclusion: This study shows that repeated 10-mg oral doses of zolpidem during 8 days does not impair nocturnal respiratory and sleep architecture parameters or diurnal pulmonary function tests, central control of breathing, and physical performances in patients with stable COPD. (CHEST 1996; 110:1203-11)
Key words chronic obstructive pulmonary disease; control of breathing; nocturnal respiratory disorders; physical performance; pulmonary function; sleep architecture; zolpidem
Abbreviations: ABG=arterial blood gas; AI=apnea index; ANOVA=analysis of variance; [P.sub.0.01]=mouth occlusion pressure; PFT=pulmonary function test; PSR=polysomnographic recording; RDI=respiratory disturbance index; REM=rapid eye movement; Sa[O.sup.2]=arterial oxygen saturation; SAS=sleep apnea syndrome; TST=total sleep time; VAS=visual analog scale
The sleep of patients with COPD is of poor quality.[1-3] Nocturnal EEG recordings demonstrate modifications of the sleep parameters, namely a decrease in the total sleep time (TST), a decrease in the duration of slow-wave and rapid eye movement (REM) sleep, and an increase in the duration of stage 1 and stage 2 sleep and of intrasleep awakenings. At the same time, recording of the nocturnal arterial oxygen saturation (Sa[O.sup.2]) curve shows the presence of hypoxic periods. These peaks of desaturation can reach 25 to 40% of baseline Sa[O.sub.2].[3] These hypoxic episodes are linked to physiologic alveolar hypoventilation, which in COPD patients are responsible for impairment of preexisting hypoxemia. These patients frequently ask for the prescription of hypnotics classically contraindicated for the stage of chronic respiratory insufficiency with hypoxemia. However, most of these hypnotics are benzodiazepines that can alter respiration during sleep by several mechanisms:[4] decrease in the response to hypoxic and hypercapnic stimuli; alteration of awakening induced by obstruction of the upper airways, hypercapnia, or hypoxia; changes in sleep architecture with increase in the duration of the stages in which respiratory abnormalities are more frequent and pharyngeal myorelaxation increasing the risk of the occurrence of obstructive events.
Zolpidem, the first imidazopyridine with a hypnotic action close to the benzodiazepines in fact binds selectively to omega[1] receptors,[5,6] and thus exhibits specific hypnotic properties.[7] Its low affinity for other benzodiazepine receptors might explain its lower myorelaxant effect[6,8,9] and its lack of respiratory depressor activity.[4] Its hypnotic efficacy has been proved by oral administration at the dose of 10 mg.[10-13] Its short plasmatic half-life results in easy awakening and an absence of diurnal residual effects.[13] Its long-term prescription does not lead to habituation or a rebound effect of insomnia.[14]
Studies of nocturnal respiratory function have been carried out on healthy subjects[4,12,15,16] and have not shown any significant respiratory disorders to be induced by zolpidem. Studies carried out on simple snoring patients have not shown significant alteration of nocturnal respiratory function at the single dose of 10 mg.[17] In contrast, in patients with sleep apnea syndrome (SAS), a nonsignificant impairment of nocturnal respiratory events has been found at the single dose of 20 mg.[18] In COPD patients, no deleterious effect of zolpidem, 10 mg, has been shown on diurnal respiratory function after a single dose[19] or repeated administration.[20] It therefore seemed interesting to evaluate the effects of continuously administered zolpidem on the nocturnal respiratory parameters of these patients.
The objective of this study was thus to assess the effects of repeated 10-mg oral doses of zolpidem, compared with placebo, on the diurnal and nocturnal respiratory function, as well as on the diurnal vigilance and physical performance of COPD patients exhibiting disordered sleep.
MATERIALS AND METHODS
The study was conducted in outpatients of the Respiratory Medicine Department and was approved by the Ethical Committee of the Charles Nicolle University Hospital. All patients had to give their written, informed consent. Eligible patients had to meet the following criteria for inclusion: (1) to have stable COPD defined as follows: FE[V.sub.1] less than 1.5 L, FE[V.sub.1]/VC less than 50%, Pa[O.sub.2] less than 75 mm Hg, PaC[O.sub.2] more than 45 mm Hg, and no acute exacerbation in the 3 last months before entry; (2) to exhibit disordered sleep that did not start before COPD; (3) not to be overweight, with a weight not differing more than 25% from the ideal theoretical weight calculated for height and age; and (4) to have a normal biological status, ie, hematologic, biochemical, and renal and liver function test results in the normal range of the laboratory. In addition, patients had not to have taken any hypnotic or psychotropic treatment within 3 weeks before inclusion, except for products with a short half-life (2 to 3 h), which had not to have been taken within 1 week before entry. Subjects were not included if they were enrolled in another therapeutic trial in the 3 last months and if they presented with cardiac, hepatic, renal, neurologic, or progressive psychiatric disease associated with COPD. We also excluded subjects having exhibited arsenal blood gas (ABG) changes of more than 15% in the 2 days preceding the study, as well as those pregnant or within the fertile period without contraception. Subjects who were alcohol or drug dependent or allergic to zolpidem or benzodiazopines were also excluded.
Study Protocol
This prospective controlled study was performed single blinded, comparing zolpidem taken orally at the dose of 10 mg/d with a placebo, each patient acting as his own control. Treatment was presented in individual blister packs of 9 tablets, numbered from 1 to 9, corresponding to each of the study nights (N1 to N9). Tablets of zolpidem and placebo were identical in presentation and were not identifiable. Tablets 1 and 9 contained the placebo and those numbered from 2 to 8 contained the 10-mg dose of zolpidem. The medication was taken at the patient's bedside on retiring at lights out. All other psychotropic treatment was forbidden during the study, as well as alcoholic drinks and coffee. The usual treatments of the patients had to be prescribed at stable doses for the entire duration of the teal.
A medical selection visit, mainly consisting of biological tests, allowed patients to be declared eligible for the study. If eligible, the patient received a "sleep diary" concerning its quality of sleep and awakening, its daytime vigilance scored by visual analog scales (VAS). Patients were required to fill in this diary each day for the 4 weeks preceding the start of the study, thus allowing evaluation of understanding and ability to do so during the trial.
For each patient, the duration of the study was 11 days (D0 to D10), ie, 10 nights (N0 to N9). On D0, the patients meeting the clinical and biological (selection visit) inclusion criteria and having adequately filled in their sleep diary during the 4 weeks were enrolled in the study after having given their written informed consent.
Measurements
On D0 and D8, included patients underwent biological tests (hematologic, biochemical, renal and liver function tests) with control of theophylline level and alcoholemia. Pulmonary function tests (PFT), study of central control of breathing pattern with hypercapnic and hypoxic stimulation, and measurement of mouth occlusion pressure in 1 s (P[sub.0.01]) were performed on D0 and D9. These parameters were recorded in sitting positron and the equipment used has been described previously.[21] Ventilatory responses to [CO.sup.2] (V/Pa[CO.sub.2]) and to hypoxia (V/Sa[O.sub.2]) were measured from linear regression lines, using respectively, the "rebreathing" method of Read[22] and the method of Rebuck and Campbell.[23] At the same fume, Pool was calculated using a standardized technique.[24,25] A 6-min walking test assessing physical performance was also performed on the same days before and after treatment (D0 and D9).
Nocturnal polysomnographic recordings (PSRs) were carried out in ambient air from 10:30 PM to 6:30 AM during nights N0, N1, N2, N8, and N9. The first night N0 was a night of habituation to recording conditions and permitted those patients with obvious abnormalities to be excluded. Nocturnal PSR consisted of an EEG (SPZ-CZ; CZ-OZ), an electro-oculogram, a submental electromyogram, and an ECG. Respiratory parameters consisted of transcutaneous nocturnal oximetry (Biox 3700; Ohmeda Inc, Boulder, Colo), recording of nasobuccal airflow by thermistance, and abdominothoracic respiratory movements by constraint gauge bands. ABG valves in ambient air were also sampled before retiring and after each nocturnal PSR on awakening (N0, N1, N2, N8, and N9). All sleep and nocturnal respiratory function recordings were examined by independent observers. Sleep architecture was analyzed using the criteria of Rechtschaffen and Kales.[26] Apnea was defined as a pause of nasobuccal airflow lasting 10 s or more. It was considered as obstructive if thoracoabdominal movements persisted and as central if they were abolished. Hypopnea (reduction of nasobuccal airflow of at least 50% for at least 10 s) was taken into account only if it was associated with a 4% or more drop in Sa[O.sub.2] relative to baseline Sa[O.sub.2]. The apnea index (AI=number of apneas per hour of sleep) was considered abnormal if it was greater than 5, and the respiratory disturbances index (RDI=number of apneas and hypopneas per hour of sleep) was considered abnormal if it was greater than 10.27 In addition, every day from D0 to D10, patients had to fill in their personal study sleep diary in which were noted the times of retiring and awakening, the estimated duration of sleep, and the phases of nocturnal awakenings and diurnal somnolence. They also evaluated in this diary their quality of sleep, morning sensation of being rested, and their mood and state of vigilance during the day by means of 100 mm VAS. The patient's status was located between a value of 0 (least good) to 100 (best) for each of the following items: quality of sleep, lucidity, dynamism, alertness, activity, and mood during the day. Intercurrent events were also reported in this notebook.
Evaluation and Statistical Analysis
The main evaluation criteria were nocturnal respiratory and sleep architecture parameters during the 4 recording nights (N1, N2, N8, and N9). The secondary criteria concerned the subjective evaluation of the quality of sleep, mood, and diurnal vigilance. Autoevaluation scores obtained with VAS were then converted into numeric values for statistical analysis. We also analyzed pulmonary function and central control of breathing during the daytime, as well as physical performance and tolerance to zolpidem, 10 mg.
Results were expressed as mean+SD and/or range values. For respiratory and sleep parameters, the changes observed between night N1 (placebo), the nights with effective treatment (N2 to N8) and the night posttreatment N9 (placebo) were studied by analysis of v variance (ANOVA) with two factors (subject, day). During a significant "day" effect, Dunnetts' multiple comparisons test was performed to determine the level of the differences relative to the baseline value. The changes before and after treatment in PFT central control of breathing, walking test, and biological variables were analyzed by a Student's tt test for paired series. A significant value for the different comparisons was set as p<0.05.
RESULTS
Twelve patients were initially enrolled in this study, but two had to be excluded after the treatment onset. One was removed on D2 because of the occurrence of a dyspnea attack with sweating on D0 (before taking zolpidem), followed by a second identical episode on D2. This patient had severe advanced COPD, not tolerating any interruption of nasal oxygen therapy. The second patient decided to stop treatment on D3. Ten patients, 56.8 [+ or -] 8.3 years old, completed the study and were finally analyzed. Their general characteristics are shown in Table 1. One patient had a history of nonprogressive depressive syndrome. All patients had associated treatments consisting essentially of theophylline, [beta]2-mimetics, inhaled corticosteroids, and bronchial fluidizers. Two subjects regularly consumed alcohol (one glass a day) and seven were smokers (Table 1). The pulmonary and sleep characteristics of the 10 COPD patients are summarized in Table 2. All had moderate hypoxemia and hypercapnia without pulmonary overdistention. Nine of them were placed on a regimen of long-term nasal oxygen therapy (1 to 3 L/min for a duration [greater than or equal to] 15 h/d). Six patients (60%) normally snored ("occasionally" in 4 patients, and "every night" in 2 patients). Snoring was regular and slight in five patients, loud and intermittent in one patient. Five patients (50%) had leg movements during sleep. Eight (80%) exhibited diurnal somnolence, but none reported nocturnal respiratory arrest.
[TABULAR DATA 5 & 6 OMITTED]
Clinically, only one patient complained of digestive disturbances, as moderate epigastric pains and heaviness, lasting for 1 to 4 h/d over the D3 to D10 period. Comparison of biological data before and after treatment showed no side effects of zolpidem, 10 mg, on hematologic, biochemical, and renal and liver function test results. Theophylline level also did not change between D0/N0 (9.88 [+ or -] 4.93 ,[mu]g/mL) and D8/N8 (9.08 [+ or -] 5.70 [mu]g/mL).
DISCUSSION
The effects of zolpidem on diurnal or nocturnal respiratory function have been studied until now in young[12,15,28,29] or elderly[4,16] healthy subjects, in healthy snoring subjects,[17] or subjects with SAS,[18] but rarely in COPD patients.[19,20] Patients with COPD often complain of insomnia[2] because of poor quality of sleep.) 3 In addition, nocturnal respiratory disorders often are associated with COPD[1] and may result in the "overlap syndrome" combining SAS and CoPD.[30] Hypnotics, the treatment of choice for insomnia, are widely taken on a regular basis in the general population.[31] Hypnotic benzodiazopines, widely used for this indication, are well known for their depressor effect on central control of breathing[32-34] and their myorelaxant effect. These two effects can therefore easily aggravate or favor the occurrence of nocturnal respiratory disorders, especially obstructive apnea and nocturnal desaturation. Moreover, these adverse effects have been clearly demonstrated in COPD patients.[19,35,36] It is thus useful to have currently available new nonbenzodiazepine hypnotics such as zolpidem. Its pharmacologic properties make it of special interest in high-risk populations like COPD patients. However, only a few studies have examined the effects of zolpidem on nocturnal respiratory parameters and none, to our knowledge, have evaluated its effects in repeated administration in COPD patients.
The present study shows that repeated 10-mg oral doses of zolpidem in the evening for 7 nights does not alter the nocturnal respiratory parameters of oxygenation and ventilation and has no effect on the physiologic sleep architecture of patients with stable mild to severe COPD. It also shows that the continuous intake of zolpidem causes subjective improvement in the quality of sleep reported by these patients and has no effect on their mood and diurnal vigilance. In addition, it produces no change in PFT results and central control of breathing during the daytime.
Our results on nocturnal respiratory function and sleep architecture are comparable to those reported in young[15] or elderly[4,16] healthy subjects. They are also similar to those reported in other populations at risk, including heavy snorers[17] or patients with moderate isolated SAS.[18] In our study, analysis of the sleep parameters showed only a significant increase in the duration of stage 2 with zolpidem. It should also be noted that, despite the lack of significant difference, several of these parameters tended to worsen when treatment was stopped (sleep latency of stage 1 and 2, REM sleep latency, intrasleep awakenings, sleep efficiency index, TST, and stage 3 to 4 duration). In their controlled-placebo study in nonapneic heavy snorers, Quera-Salva et al[17] also found a significant increase in the duration of stage 2, as well as an increase in TST, in sleep efficiency index and a decrease in the duration of in trasleep awakenings, and therefore a hypnotic effect after a single dose of zolpidem, 10 mg. Similarly, zolpidem, 20 mg, and flurazepam, 30 mg, in a single dose in SAS patients, increased TST and the sleep efficiency index with a decrease in sleep latency, intrasleep awakenings, and the number of awakenings without change in the different sleep stages.[13] In contrast, no modification of sleep architecture was found in healthy young subjects after a single dose of zolpidem, 10 mg,[15] or in healthy elderly subjects after 7 nights of treatment with the same dose.[16] At the same time in our study we found, as others had, a subjective improvement in the quality of sleep[16] without any change in mood or in parameters of vigilance and without diurnal residual effect after treatment with zolpidem.[13,17,37,33] It should again be noted that, despite the lack of significant difference, some parameters assessing vigilance (lucidity, dynamism, alertness) and mood worsened in our patients when treatment was stopped.
As regards the nocturnal respiratory parameters, we found no significant difference in the RDI or the different Sa[O.sub.2] parameters. When compared with the diurnal ABG data, the oxygenation parameters during the different recording nights prove nocturnal impairment of physiologic alveolar hypoventilation in COPD patients, responsible for a nocturnal hypoxemia which did not seem to be increased herein by repeated doses of zolpidem. The RDI, already abnormal on inclusion in our patients, proves that there were some with moderate sleep respiratory disorders. These disorders, often associated in COPD [1,3,30] are especially prone to aggravation by intake of hypnotics.[35,36] Indeed, six of our patients had "overlap syndrome,"[30] two predominantly apneic and four predominantly hypopneic.[39] However, these nocturnal respiratory disorders were not increased by treatment as compared with placebo. In contrast, it has been shown that zolpidem as a single 20-mg dose, ie, twice the normal recommended therapeutic dose, induced a nonsignificant increase in the RDI, compared to placebo in a middle-aged SAS population.[18] Nevertheless, this was associated with a significant decrease both of mean and minimum Sa[O.sub.2] during apneic events compared with placebo and with flurazepam. These results, moreover, led the authors to contraindicate zolpidem at this dose for patients with obstructive SAS. Simple snorers[40] and elderly subjects[41,42] are also known to be populations at a higher risk of nocturnal respiratory disorders able to be induced or impaired by intake of hypnotics.[34,43] It has been shown in a population of simple snorers that zolpidem, as a single 10-mg dose compared with placebo, significantly increased the RDI which remained, however, below 5 during treatment, except in 1 patient in whom it changed from 3 to 10 per hour of sleep.[17] These same authors also reported a lower minimum Sa[O.sub.2] after zolpidem but considered as more representative of nocturnal desaturation the percentage of TST spent at an Sa[O.sub.2] of less than 90% and with a decrease less than 4% relative to baseline Sa[O.sub.2], values that remained statistically unchanged. They furthermore concluded that zolpidem, 10 mg, had no significant deleterious effect on respiratory function in such patients. In elderly subjects, a study performed in 10 women after orthopedic surgery evaluated the effects of zolpidem, 10 mg, administered for 4 consecutive nights on nocturnal respiratory function assessed by simple inductance plethysmography and transcutaneous oximetry.[4] There was no difference in the incidence of nocturnal respiratory disorders between the treated and placebo groups during the different nights. In contrast, the frequency of apnea inducing a drop in Sa[O.sub.2] less than 90% was found to be greater during the first night than during the last night of treatment. These results are similar to those of another study which found, in 12 elderly subjects, a significant increase in the AI during slow-wave and REM sleep after the first dose of zolpidem, 10 mg, but this index then decreased after repeated administration.[16] Our results on nocturnal respiratory function and sleep parameters are also comparable to those obtained with COPD patients[21,44] or moderate SAS[45] receiving Zopiclone at the dose of 7.5 mg. Zopiclone is another recently discovered hypnotic with pharmacologic properties close to those of benzodiazepines, but short-acting[46,47] and devoid of respiratory depressor effect in the healthy subject.[48] However, it seems that this molecule causes significant worsening of the AI and nocturnal desaturation in elderly patients with severe SAS.[49]
In our study, we also analyzed ABG sampled on retiring and awakening, as well as PFT, central control of breathing, and physical walking performances during daytime. No significant change in ABG values was observed on awakening during treatment relative to the baseline values. In contrast, moderate impairment of diurnal alveolar hypoventilation was found in ABG sampled on retiring. This alveolar hypoventilation was associated with diurnal hypoxemia on retiring only during the eighth night (D8/N8). These surprising results are difficult to explain insofar as they appeared only episodically and discordantly depending on the night, as there was no impairment of nocturnal alveolar hypoventilation, since the hypoxemia, reflected by Sa[O.sub.2] parameters, was not altered during treatment. Finally, we would expect to see, in this context, more an aggravation of this hypoventilation rather on awakening than on retiring. In addition, it could not be a precocious effect of zolpidem, since ABG determinations on retiring were performed before the tablet was taken. Also, it could not be a "new release" effect of the drug because of its short half-life of elimination (2.5 to 3.5 h) and its lack of active metabolites.[50] These gasometric observations on retiring are therefore very probably related to individual variations in ABG determinations from one sampling to another in patients with mild to severe COPD. Moreover, it has been clearly shown that zolpidem, 10 mg, does not change diurnal ABG values in patients with severe COPD 2 h after a single dose[19] and even after repeated administration.[20]
In addition, we did not observe any change in PFT results, breathing pattern, and central control of breathing data after 1 week of treatment compared with placebo. These results confirm those of previous studies specifically assessing the influence of zolpidem, 10 mg, on control of breathing after a single dose[19] or long-term[20] in patients with severe COPD. That confirms the safe use of zolpidem in such patients whose central respiratory centers, in contrast, prove to be sensitive to benzodiazepines.[19] However, it should be noted that it has been shown, in healthy subjects, that even though zolpidem does not globally alter central respiratory drive, it nevertheless induces a slight but significant decrease in the duration of the respiratory cycle, as regards the inspiratory time and total cycle time.[28] It should be noted that this was not found in our study and that some of our patients could not tolerate one or other of the hypoxic and hypercapnic stimulation tests. This study also shows that zolpidem does not affect the physical performance in COPD patients. After treatment, our patients could cover a longer distance in the walking test, but this was not significantly different compared with placebo. This finding confirms the data reported in young athletes in whom zolpidem, 10 mg, had no deleterious effect on diurnal physical performance contrary to sleep deprivation.[29]
Regarding biological tolerance, no significant side effects were noted after 1 week of treatment. Similarly, zolpidem seems to not interact with the theophylline level which remained stable during the whole period trial. Clinically, only one patient complained of digestive disturbances. This could not be judged treatment unrelated, as occasional digestive disturbances have already been reported with zolpidem.[51]
In conclusion, compared with placebo, the repeated evening administration of 10-mg oral doses of zolpidem in patients with stable COPD does not impair nocturnal respiratory function and has no deleterious effect on physiologic sleep architecture. In addition, zolpidem improves subjective quality of sleep and does not alter either mood or vigilance in these patients. It also has no effect on daytime PFT results, breathing pattern, and central control of breathing. Its tolerance also proved to be completely satisfactory in this population.
ACKNOWLEDGMENTS: We would like to thank Laboratoire Synthelabo, Division SNC, for their logistic and statistical help in performing this study.
REFERENCES
[1] Fleetham J, West P, Mezon B, et al. Sleep, arousals and oxygen desaturation in chronic obstructive pulmonary disease. Am Rev Respir Dis 1982, 126:429-33 [2] Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airway diseases. Chest 1987; 91:540-46 [3] Muir JF. Le sommeil du bronchitique chronique. Rev Prat 1989; 39:581-83 [4] Rhodes SP, Parry P, Hanning CD. A comparison of the effects of zolpidem and placebo on respiration and oxygen saturation during sleep in the healthy elderly. Br J Clin Pharmacol 1990; 30:817-24 [5] Langer SZ, Arbilla S, Scatton B, et al. Receptors involved in the mechanism of action of zolpidem. In: Sauvanet JP, Langer SZ, Morselli PL, eds. Imidazopyridines in sleep disorders: a novel experimental and therapeutic approach. New York: Raven Press, 1988; 55-70 [6] Lloyd KG, Zivkovic B. Specificity within the GABA receptor supramolecular complex: a consideration of the new omega 1 receptor selective imidazopyridine hypnotic zolpidem. Pharmacol Biochem Behav 1988; 129:781-83 [7] Zivkovic B, Perrault G, Morel E, et al. Comparative pharmacology of zolpidem and other hypnotics and sleep inducers. In: Sauvanet JP, Langer SZ, Morselli PL, eds. Imidazopyridines in sleep disorders: a novel experimental and therapeutic approach, New York: Raven Press, 1988; 97-109 [8] Depoortere H, Zivkovic B, Lloyd KG, et al. Zolpidem, a novel non-benzodiazepine hypnotic: neuropharmacological and behavioral effects. J Pharmacol Ther 1986; 237:649-58 [9] Sanger DJ, Perrault G, Morel E, et al. The behavioral profile of zolpidem: a novel drug of imidazopyridine structure. Physiol Behav 1987; 41:23540 [10] Nicholson AN, Pascoe PA. Hypnotic activity of an imidazopyridine (zolpidem). Br J Clin Pharmacol 1986; 21:205-11 [11] Herrmann WM, Kubicki S, Wober W. Zolpidem: a 4-week pilot polysomnographic study in patients with chronic sleep disturbances. In: Sauvanet JP, Langer SZ, Morselli PL, eds. Imidazopyridines in sleep disorders: a novel experimental and therapeutic approach. New York: Raven Press, 1988; 261-78 [12] Merlotti L, Roehrs T, Khoshorek G, et al. The dose effects of zolpidem on the sleep of healthy normals. J Clin Psychopharmacol 1989; 9:9-14 [13] Bensimon G, Foret J, Warot D, et al. Daytime wakefulness following a bedtime oral dose of zolpidem 20 mg. Br J Clin Pharmacol 1990; 30:463-69 [14] Schlich D, L'Heritier C, Coquelin JP, et al. Long-term treatment of insomnia with zolpidem 20 mg. multicentre general practitioner study of 107 patients. J Int Med Res 1991; 19:271-79 [15] Crowe McCann C, Quera-Salva MA, Boudet J, et al. Effect of zolpidem during sleep on ventilation and cardiovascular variables in normal subjects. Fundam Clin Pharmacol 1993; 7:305-10 [16] Kurtz D, Fillius B, Boningen C, et al. Effect of zolpidem on the sleep of healthy elderly subjects: polysomnographic recording assessments and pharmacokinetics. In Sauvanet JP, Langer SZ, Morselli PL, eds. Imidazopyridines in sleep disorders: a novel experimental and therapeutic approach. New York: Raven Press, 1988; 382 [17] Quera-Salva MA, Crowe McCann C, Boudet J, et al. Effects of zolpidem on sleep architecture, nighttime ventilation, daytime vigilance and performance in heavy snorers. Br J Clin Pharmacol 1994; 37:539-43 [18] Cirignotta F, Mondini S, Zucconi M, et al. Zolpidem-polysomnographic study of a new hypnotic drug in sleep apnea syndrome. Pharmacol Biochem Behav 1988; 29:807-09 [19] Murciano D, Armengaud MH, Cramer PH, et al. Acute effects of zolpidem, triazolam, flunitrazepam on arsenal blood gases and control of breathing in severe COPD. Eur Respir J 1993; 6:625-29 [20] Murciano D, Moreau J, Barthouil P, et al. Long-term effects of zolpidem (z) on arsenal blood gases and control of breathing in patients with severe chronic obstructive pulmonary disease [abstract]. J Sleep Res 1992; 1(suppl 1):156 [21] Muir JF, Defouilloy C, Broussier P, et al. Comparative study of the effects of zopiclone and placebo on respiratory function in patients with chronic respiratory insufficiency. Int Clin Psychopharmacol 1990; 5:85-94 [22] Read DJC. A clinical method for assessing the ventilatory response to carbon dioxide. Aust Ann Med 1967; 16:20-32 [23] Rebuck AS, Campbell EJ. A clinical method for assessing the ventilatory response to hypoxia. Am Rev Respir Dis 1974; 109:345-50 [24] Whitelaw WA, Derenne JP, Milic-Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol 1975; 23:181-99 [25] Aubier M, Murciano D, Fournier M, et al. Central respiratory drive in acute respiratory failure of patients with chronic obstructive lung disease. Am Rev Respir Dis 1980; 122:191-99 [26] Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages in human subjects. Washington, DC: National Institute of Public Health, publication 204, 1986 [27] American Thoracic Society, Medical Section of the American Lung Association. Indications and standards for cardiopulmonary sleep studies. Am Rev Respir Dis 1989; 139:559-68 [28] Maillard D, Thiercelin JF, Fuseau E, et al. Effects of zolpidem versus diazepam and placebo on breathing control parameters in healthy human subjects. Int J Clin Pharmacol 1992; 12:27-35 [29] Mougin F, Simon-Rigaud ML, Davenne D, et al. Tolerance a l'effort apres reduction de sommeil et prise d'un hypnotique: le zolpidem. Arch Int Physiol Biochim Biophys 1992; 100:255-62 [30] Flenley DC. Sleep in chronic lung disease. Clin Chest Med 1986; 6:651-58 [31] Quera-Salva MA, Orluc A, Goldenberg F, et al. Insomnia and use of hypnotics: study of a French population. Sleep 1991; 14:386-91 [32] Catchlobe RHF, Kafer ER. The effects of diazepam on the ventilatory response to carbon dioxide and on the steady-state gas exchange. Anesthesiology 1971; 34:9-18 [33] Cohn MA. Hypnotics and the control of breathing: a review. Br J Clin Pharmacol 1983; 16:245S-50S [34] Dolly FR, Block AJ. Effect of flurazepam on sleep-disordered breathing and nocturnal oxygen desaturation in asymptomatic patients. Am J Med 1982; 73:239-43 [35] Block AJ, Dolly FR, Slayton PC. Does flurazepam affect breathing and oxygenation during sleep in patients with chronic lung disease? Am Rev Respir Dis 1984; 129:230-33 [36] Timms R, Dawson A, Hajdukovic RM, et al. Effect of triazolam on sleep and arterial oxygen saturation in patients with chronic obstructive pulmonary disease. Arch intern Med 1988; 148: 2159-63 [37] Besset A, Tafti M, Barthouil P, et al. Effect of zolpidem 10 mg on sleep architecture and daytime psychomotor skills in poor sleepers. Sleep Res 1990; 19:54 [38] Dujardin K, Derambure P, Guieu JD, et al. Effects of zolpidem 10 mg on cognitive performance: an extensive investigation [abstract]. J Sleep Res 1992; 1(suppl 1):64 [39] Gould G, Whyte G, Airies M, et al. The sleep hypopnea syndrome. Am Rev Respir Dis 1988; 137:895-98 [40] Norton PG, Dunn EV. Snoring as a risk factor for disease: an epidemiological survey. BMJ 1985; 291:630-32 [41] Ancoli-Israel S, Kripke D, Mason W. Characteristics of obstructive and central sleep apnea in the elderly: an interim report. Biol Psychiatry 1987; 22:741-50 [42] Bliwise D, Feldman D, Bliwise N, et al. Risk factors for sleep disorder breathing in heterogeneous geriatric populations. J Am Geriatr Soc 1987; 35:132-41 [43] Fancourt G, Castleden M. Use of benzodiazepines with particular reference to the elderly. Br J Hosp Med 1986; 35:321-26 [44] Molle J, Quarre J, Gilbert P, et al. Influence of zopiclone on nocturnal oxygen saturation in patients with mild chronic obstructive pulmonary disease. Sleep Res 1991; 20A:161 [45] Wildschiodtz G, Gynning B. The effect of zopiclone on sleep in patients with mild sleep apnea [abstract]. Sleep Res 1991; 20A:184 [46] Julou L, Bartone MC, Blanchard JC, et al. Pharmacological studies on zopiclone. Pharmacology 1983; 27(suppl 2):46-58 [47] Goa K, Heel R. Zopiclone: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy as a hypnotic. Drugs 1986; 32:48-65 [48] Ranlov P, Nielsen S. The effects of zopiclone and diazepam on ventilatory response in normal subjects. Sleep 1987; 10(suppl 1):40-7 [49] Inoue Y, Komatsu K, Takata K, et al. Comparison of zopiclone and flurazepam on sleep apnea in the elderly [abstract]. Sleep Res 1991; 20A:325 [50] Thenot JP, Hermann P, Durand A, et al. Pharmacokinetics and metabolism of zolpidem in various animal species and in humans. In: Sauvanet JP, Langer SZ, Morselli PL, eds. Imidazopyridines in sleep disorders: a novel experimental and therapeutic approach. New York: Raven Press, 1988; 139-53 [51] Garnier R, Guerault E, Muzard D, et al. Acute zolpidem poisoning--analysis of 344 cases. Clin Toxicol 1994; 32:391-404
(*) From the Service de Pneumologie, Hopital de Boisguillaume Centre Hospitalier Universitaire, Rouen Cedex, France (Drs. Girault, Muir, and Mihaltan); Laboratoire Synthelabo, Division SNC Meudon-la Foret Cedex, France (Drs. Borderies and De La Giclais); and Service d'Explorations Neurophysiologiques, Hopital Charles Nicolle, Centre Hospitalier Universitaire, Rouen Cedex France (Drs. Verdure and Samson-Dollfus). Manuscript received March 6, 1996; revision accepted June 11. Reprint requests: Dr. Girault, Service de Pneumologie, Hopital de Boisguillaume, Centre Hospitalier Universitaire, 76031 Rouen Cedex, France
COPYRIGHT 1996 American College of Chest Physicians
COPYRIGHT 2004 Gale Group