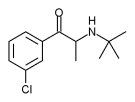
Zyban
Bupropion (amfebutamone) is an antidepressant of the amino ketone class, chemically unrelated to tricyclics or selective serotonin reuptake inhibitors (SSRIs). It is similar in structure to the stimulant cathinone, and to phenethylamines in general. It is a chemical derivative of diethylpropion, an amphetamine-like substance used as an anorectic. Bupropion is both a dopamine reuptake inhibitor and a norepinephrine reuptake inhibitor. more...
History
Bupropion was first synthesized by Burroughs Research in 1966, and patented by Burroughs-Wellcome (later Glaxo-Wellcome, and, as of 2000, GlaxoSmithKline) in 1974. It was approved by the FDA in 1985 and marketed under the name Wellbutrin as an antidepressant, but clinical trials indicated that incidence of seizure was two to four times greater than other antidepressants and the drug was quickly pulled from the market. It was subsequently discovered that reducing the dose by about half greatly reduced the risk of seizures. Glaxo then developed a sustained-release (SR) version of Wellbutrin which releases bupropion hydrochloride at a slower rate. The SR formulation is taken twice a day, in order to further decrease the possibility of adverse side effects and seizures. It is also available in generic form (Bupropion SR). Extended Release bupropion, Wellbutrin XL, is the most recent formulation of bupropion and is taken orally once a day. Because of this altered mechanism of delivery and reduced dosing, incidence of seizures with bupropion is comparable to, and in some cases, lower than that of other antidepressants.
In 1997, bupropion HCl was approved by the FDA for use as a smoking cessation aid. Glaxo subsequently marketed the drug under the name Zyban to help people stop smoking tobacco by reducing the severity of craving and addiction/withdrawal symptoms. It can be used in combination with nicotine replacement therapies. Bupropion treatment course lasts for seven to twelve weeks, with the patient halting the use of tobacco around ten days into the course.
Bupropion is also being investigated as a weight loss drug.
Mode of action
Bupropion is a selective catecholamine (norepinephrine and dopamine) reuptake inhibitor. It has only a small effect on serotonin reuptake. It does not inhibit MAO. The actual mechanism behind bupropion's action is not known, but it is thought to be due to the effects on dopaminergic and noradrenergic mechanisms.
Pharmacokinetics
Bupropion is metabolised in the liver. It has at least three active metabolites: hydroxybupropion, threohydrobupropion and erythrohydrobupropion. These active metabolites are further metabolised to inactive metabolites and eliminated through excretion into the urine. The half-life of bupropion is 20 hours as is hydroxybupropion's. Threohydrobupropion's half-life is 37 hours and erythrohydrobupropion's 33 hours.
Chronic hepatotoxicity in animals
In rats receiving large doses of bupropion chronically, there was an increase in incidence of hepatic hyperplastic nodules and hepatocellular hypertrophy. In dogs receiving large doses of bupropion chronically, various histologic changes were seen in the liver, and laboratory tests suggesting mild hepatocellular injury were noted.
Read more at Wikipedia.org